Abstract
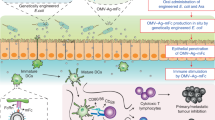
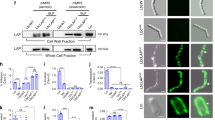
We recently demonstrated that noninvasive food-grade Lactococcus lactis (L. lactis) can deliver eukaryotic expression plasmid in mammalian cells in vitro. Here, we evaluated, in vivo, whether a eukaryotic expression plasmid carried by lactococci can translocate to the epithelial cells of the intestinal membrane. The strain LL(pLIG:BLG1) carrying one plasmid containing a eukaryotic expression cassette encoding β-lactoglobulin (BLG), a major allergen of cow's milk, was orally administered by gavage to mice. BLG cDNA was detected in the epithelial membrane of the small intestine of 40% of the mice and BLG was produced in 53% of the mice. Oral administration of LL(pLIG:BLG1) induced a low and transitory Th1-type immune response counteracting a Th2 response in case of further sensitization. We demonstrated for the first time the transfer of a functional plasmid to the epithelial membrane of the small intestine in mice by noninvasive food-grade lactococci.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Abbreviations
- LL:
-
Lactococcus lactis MG1363
- PBS:
-
phosphate buffer saline
- EIA:
-
enzyme immuno assay
References
Hanniffy S, Wiedermann U, Repa A, Mercenier A, Daniel C, Fioramonti J et al. Potential and opportunities for use of recombinant lactic acid bacteria in human health. Adv Appl Microbiol 2004; 56: 1–64.
Nouaille S, Ribeiro LA, Miyoshi A, Pontes D, Le Loir Y, Oliveira SC et al. Heterologous protein production and delivery systems for Lactococcus lactis. Genet Mol Res 2003; 2: 102–111.
Guimaraes VD, Innocentin S, Lefevre F, Azevedo V, Wal JM, Langella P et al. Use of native lactococci as vehicles for delivery of DNA into mammalian epithelial cells. Appl Environ Microbiol 2006; 72: 7091–7097.
Schoen C, Stritzker J, Goebel W, Pilgrim S . Bacteria as DNA vaccine carriers for genetic immunization. Int J Med Microbiol 2004; 294: 319–335.
Dunham SP . The application of nucleic acid vaccines in veterinary medicine. Res Vet Sci 2002; 73: 9–16.
Gaillard JL, Berche P, Frehel C, Gouin E, Cossart P . Entry of L. monocytogenes into cells is mediated by internalin, a repeat protein reminiscent of surface antigens from gram-positive cocci. Cell 1991; 65: 1127–1141.
Isberg RR, Falkow S . A single genetic locus encoded by Yersinia pseudotuberculosis permits invasion of cultured animal cells by Escherichia coli K-12. Nature 1985; 317: 262–264.
Grillot-Courvalin C, Goussard S, Huetz F, Ojcius DM, Courvalin P . Functional gene transfer from intracellular bacteria to mammalian cells. Nat Biotechnol 1998; 16: 862–866.
Guimaraes VD, Gabriel JE, Lefevre F, Cabanes D, Gruss A, Cossart P et al. Internalin-expressing Lactococcus lactis is able to invade small intestine of guinea pigs and deliver DNA into mammalian epithelial cells. Microbes Infect 2005; 7: 836–844.
Lecuit M, Ohayon H, Braun L, Mengaud J, Cossart P . Internalin of Listeria monocytogenes with an intact leucine-rich repeat region is sufficient to promote internalization. Infect Immun 1997; 65: 5309–5319.
Schaffner W . Direct transfer of cloned genes from bacteria to mammalian cells. Proc Natl Acad Sci USA 1980; 77: 2163–2167.
Cicin-Sain L, Brune W, Bubic I, Jonjic S, Koszinowski UH . Vaccination of mice with bacteria carrying a cloned herpesvirus genome reconstituted in vivo. J Virol 2003; 77: 8249–8255.
Shiau AL, Chu CY, Su WC, Wu CL . Vaccination with the glycoprotein D gene of pseudorabies virus delivered by nonpathogenic Escherichia coli elicits protective immune responses. Vaccine 2001; 19: 3277–3284.
Jijon H, Backer J, Diaz H, Yeung H, Thiel D, McKaigney C et al. DNA from probiotic bacteria modulates murine and human epithelial and immune function. Gastroenterology 2004; 126: 1358–1373.
Obermeier F, Dunger N, Strauch UG, Hofmann C, Bleich A, Grunwald N et al. CpG motifs of bacterial DNA essentially contribute to the perpetuation of chronic intestinal inflammation. Gastroenterology 2005; 129: 913–927.
Rachmilewitz D, Katakura K, Karmeli F, Hayashi T, Reinus C, Rudensky B et al. Toll-like receptor 9 signaling mediates the anti-inflammatory effects of probiotics in murine experimental colitis. Gastroenterology 2004; 126: 520–528.
Palka-Santini M, Schwarz-Herzke B, Hosel M, Renz D, Auerochs S, Brondke H et al. The gastrointestinal tract as the portal of entry for foreign macromolecules: fate of DNA and proteins. Mol Genet Genomics 2003; 270: 201–215.
Hohlweg U, Doerfler W . On the fate of plant or other foreign genes upon the uptake in food or after intramuscular injection in mice. Mol Genet Genomics 2001; 265: 225–233.
Schubbert R, Hohlweg U, Renz D, Doerfler W . On the fate of orally ingested foreign DNA in mice: chromosomal association and placental transmission to the fetus. Mol Gen Genet 1998; 259: 569–576.
Niswender KD, Blackman SM, Rohde L, Magnuson MA, Piston DW . Quantitative imaging of green fluorescent protein in cultured cells: comparison of microscopic techniques, use in fusion proteins and detection limits. J Microsc 1995; 180 (Part 2): 109–116.
Negroni L, Bernard H, Clement G, Chatel JM, Brune P, Frobert Y et al. Two-site enzyme immunometric assays for determination of native and denatured beta-lactoglobulin. J Immunol Methods 1998; 220: 25–37.
Drouault S, Corthier G, Ehrlich SD, Renault P . Survival, physiology, and lysis of Lactococcus lactis in the digestive tract. Appl Environ Microbiol 1999; 65: 4881–4886.
Rochat T, Gratadoux JJ, Corthier G, Coqueran B, Nader-Macias ME, Gruss A et al. Lactococcus lactis SpOx spontaneous mutants: a family of oxidative-stress-resistant dairy strains. Appl Environ Microbiol 2005; 71: 2782–2788.
Wilcks A, van Hoek AH, Joosten RG, Jacobsen BB, Aarts HJ . Persistence of DNA studied in different ex vivo and in vivo rat models simulating the human gut situation. Food Chem Toxicol 2004; 42: 493–502.
Hazebrouck S, Pothelune L, Azevedo V, Corthier G, Wal JM, Langella P . Efficient production and secretion of bovine beta-lactoglobulin by Lactobacillus casei. Microb Cell Fact 2007; 6: 12.
Chatel JM, Adel-Patient K, Creminon C, Wal JM . Expression of a lipocalin in prokaryote and eukaryote cells: quantification and structural characterization of recombinant bovine beta-lactoglobulin. Protein Expr Purif 1999; 16: 70–75.
Stappenbeck TS, Wong MH, Saam JR, Mysorekar IU, Gordon JI . Notes from some crypt watchers: regulation of renewal in the mouse intestinal epithelium. Curr Opin Cell Biol 1998; 10: 702–709.
Adel-Patient K, Ah-Leung S, Creminon C, Nouaille S, Chatel JM, Langella P et al. Oral administration of recombinant Lactococcus lactis expressing bovine beta-lactoglobulin partially prevents mice from sensitization. Clin Exp Allergy 2005; 35: 539–546.
Chatel JM, Langella P, Adel-Patient K, Commissaire J, Wal JM, Corthier G . Induction of mucosal immune response after intranasal or oral inoculation of mice with Lactococcus lactis producing bovine beta-lactoglobulin. Clin Diagn Lab Immunol 2001; 8: 545–551.
Borrego B, Fernandez-Pacheco P, Ganges L, Domenech N, Fernandez-Borges N, Sobrino F et al. DNA vaccines expressing B and T cell epitopes can protect mice from FMDV infection in the absence of specific humoral responses. Vaccine 2006; 24: 3889–3899.
Foligne B, Zoumpopoulou G, Dewulf J, Ben Younes A, Chareyre F, Sirard JC et al. A key role of dendritic cells in probiotic functionality. PLoS ONE 2007; 2: e313.
Mohamadzadeh M, Olson S, Kalina WV, Ruthel G, Demmin GL, Warfield KL et al. Lactobacilli activate human dendritic cells that skew T cells toward T helper 1 polarization. Proc Natl Acad Sci USA 2005; 102: 2880–2885.
Macpherson AJ, Uhr T . Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science 2004; 303: 1662–1665.
Simon D, Chopin A . Construction of a vector plasmid family and its use for molecular cloning in Streptococcus lactis. Biochimie 1988; 70: 559–566.
Chatel JM, Adel-Patient K, Creminon C, Wal JM . Expression of a lipocalin in prokaryote and eukaryote cells: quantification and structural characterization of recombinant bovine beta-lactoglobulin. Protein Expr Purif 1999; 16: 70–75.
Gasson MJ . Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J Bacteriol 1983; 154: 1–9.
Langella P, Le Loir Y, Ehrlich SD, Gruss A . Efficient plasmid mobilization by pIP501 in Lactococcus lactis subsp. lactis. J Bacteriol 1993; 175: 5806–5813.
Adel-Patient K, Bernard H, Ah-Leung S, Creminon C, Wal JM . Peanut- and cow's milk-specific IgE, Th2 cells and local anaphylactic reaction are induced in Balb/c mice orally sensitized with cholera toxin. Allergy 2005; 60: 658–664.
Eum SY, Haile S, Lefort J, Huerre M, Vargaftig BB . Eosinophil recruitment into the respiratory epithelium following antigenic challenge in hyper-IgE mice is accompanied by interleukin 5-dependent bronchial hyperresponsiveness. Proc Natl Acad Sci USA 1995; 92: 12290–12294.
Adel-Patient K, Creminon C, Bernard H, Clement G, Negroni L, Frobert Y et al. Evaluation of a high IgE-responder mouse model of allergy to bovine beta-lactoglobulin (BLG): development of sandwich immunoassays for total and allergen-specific IgE, IgG1 and IgG2a in BLG-sensitized mice. J Immunol Methods 2000; 235: 21–32.
Ellman GL . The biuret reaction: changes in the ultraviolet absorption spectra and its application to the determination of peptide bonds. Anal Biochem 1962; 3: 40–48.
Acknowledgements
We thank Luis Bermudez Humaran for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chatel, JM., Pothelune, L., Ah-Leung, S. et al. In vivo transfer of plasmid from food-grade transiting lactococci to murine epithelial cells. Gene Ther 15, 1184–1190 (2008). https://doi.org/10.1038/gt.2008.59
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2008.59
Keywords
This article is cited by
-
Lactococcus lactis engineered to deliver hCAP18 cDNA alleviates DNBS-induced colitis in C57BL/6 mice by promoting IL17A and IL10 cytokine expression
Scientific Reports (2022)
-
Mucosal delivery of Lactococcus lactis carrying an anti-TNF scFv expression vector ameliorates experimental colitis in mice
BMC Biotechnology (2019)
-
In vivo delivery of pPERDBY to BALB/c mice by LacVax® DNA-I and comparison of elicited immune response with conventional immunization methods
Gene Therapy (2018)
-
Display of recombinant proteins at the surface of lactic acid bacteria: strategies and applications
Microbial Cell Factories (2016)
-
Surface display of an anti-DEC-205 single chain Fv fragment in Lactobacillus plantarum increases internalization and plasmid transfer to dendritic cells in vitro and in vivo
Microbial Cell Factories (2015)