Abstract
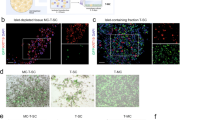
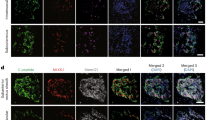
We describe the durable correction of streptozotocin-induced murine diabetes by in vivo implantation of primary mouse hepatocytes electroporated ex vivo with a human proinsulin cDNA plasmid construct controlled by glucose and zinc regulatory elements. Transfected hepatocytes increased insulin transgene transcription and secretion within 10–20 min of exposure to 25 mM glucose or 60 μM zinc. Insulin release did not occur from secretory granules. Electroporated Rosa26 hepatocytes (∼8 × 105 viable cells) were implanted in C57BL/6J diabetic mice in one of three sites: unresected liver, regenerating liver or mesentery. Control diabetic mice were implanted with untransfected hepatocytes. At 30 days after implantation, 8/15 control mice were alive, while 19/19 treated mice were alive. The ratio of body weight on day 30/nadir body weight was significantly higher for all treated groups compared with controls. All eight surviving control mice were hyperglycemic 30 days post-implantation, while 16/19 treated diabetic mice remained normoglycemic. Treated mice had lower mean glucose values (P⩽0.001) without fasting hypoglycemia and better glucose tolerance (P⩽0.0003) than untreated controls. All (6/6) diabetic mice implanted in regenerating liver and 71% (5/7) implanted in unresected liver were alive 77 days after implantation. Engrafted hepatocytes were identified, mainly around central veins, by staining for β-galactosidase activity and with anti-human insulin antibody.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kolodka TM, Finegold M, Moss L, Woo SLC . Gene therapy for diabetes mellitus in rats by hepatic expression of insulin. Proc Natl Acad Sci USA 1995; 92: 3293–3297.
Thule PM, Liu JM . Regulated hepatic insulin gene therapy of STZ-diabetic rats. Gene Therapy 2000; 7: 1744–1752.
Tuch BE et al. Function of a genetically modified human liver cell line that stores, processes and secretes insulin. Gene Therapy 2003; 10: 490–503.
Falqui L et al. Reversal of diabetes in mice by implantation of human fibroblasts genetically engineered to release mature human insulin. Hum Gene Ther 1999; 10: 1753–1762.
Liu Q, Muruve DA . Molecular basis of the inflammatory response to adenovirus vectors. Gene Therapy 2003; 10: 935–940.
Hacein-Bey-Abina S et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science 2003; 302: 415–419.
Lumelsky N et al. Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets. Science 2001; 292: 1389–1394.
Ber I et al. Functional, persistent and extended liver to pancreas transdifferentiation. J Biol Chem 2003; 287: 31950–31957.
Kojima H et al. NeuroD-betacellulin gene therapy induces islet neogenesis in the liver and reverses diabetes in mice. Nat Med 2003; 9: 596–603.
Halban PA, Kahn SE, Lernmark A, Rhodes CJ . Gene and cell-replacement therapy in the treatment of type 1 diabetes. Diabetes 2001; 50: 2181–2191.
Schwartz GP, Burke GT, Katsoyannis PG . A superactive insulin: [B10-aspartic acid] insulin (human). Proc Natl Acad Sci USA 1987; 84: 6408–6411.
Chen X, Patil JG, Lok SHL, Kon OL . Human liver-derived cells stably modified for regulated proinsulin secretion function as bioimplants in vivo. J Gene Med 2002; 4: 447–458.
Thomas G . Furin at the cutting edge: from protein traffic to embryogenesis and disease. Nat Rev Mol Cell Biol 2002; 3: 753–766.
Auricchio A et al. Constitutive and regulated expression of processed insulin following in vivo hepatic gene transfer. Gene Therapy 2002; 9: 963–971.
Burke GT et al. Superactive insulins. Biochem Biophys Res Commun 1990; 173: 982–987.
Carroll RJ et al. A mutant human proinsulin is secreted from islets of Langerhans in increased amounts via an unregulated pathway. Proc Natl Acad Sci USA 1988; 85: 8943–8947.
Itoh N, Okamoto H . Translational control of proinsulin synthesis by glucose. Nature 1980; 283: 100–102.
Welsh M, Nielsen DA, MacKrell AJ, Steiner DF . Control of insulin gene expression in β-cells and in an insulin-producing cell line, RIN-5F cells. II. Regulation of insulin mRNA stability. J Biol Chem 1985; 260: 13590–13594.
Kawaguchi T, Takenoshita M, Kabashima T, Uyeda K . Glucose and cAMP regulate the L-type pyruvate kinase gene by phosphorylation/dephosphorylation of the carbohydrate response element binding protein. Proc Natl Acad Sci USA 2001; 98: 13710–13715.
Pratley RE, Weyer C . The role of impaired early insulin secretion in the pathogenesis of type II diabetes mellitus. Diabetologia 2001; 44: 929–945.
Alam T, Sollinger HW . Glucose-regulated insulin production in hepatocytes. Transplantation 2002; 74: 1781–1787.
Castillo MB et al. Production and analysis of transgenic mice with ectopic expression of parvalbumin. Arch Biochem Biophys 1995; 317: 292–298.
Hellerstrom C, Andersson A, Gunnarsson R . Regeneration of islet cells. Acta Endocrinol Suppl (Copenh) 1976; 205: 145–160.
Olson DE et al. Glucose-responsive hepatic insulin gene therapy of spontaneously diabetic BB/Wor rats. Hum Gene Ther 2003; 14: 1401–1413.
Yasutomi K et al. Intravascular insulin gene delivery as potential therapeutic intervention in diabetes mellitus. Biochem Biophys Res Commun 2003; 310: 897–903.
Roth DA et al. Nonviral transfer of the gene encoding coagulation factor VIII in patients with severe hemophilia A. N Engl J Med 2001; 344: 1735–1742.
Bumgardner GL et al. A functional model of hepatocyte transplantation for in vivo immunologic studies. Transplantation 1998; 65: 53–61.
Zeller R . In: Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K (eds). Current Protocols in Molecular Biology. John Wiley & Sons, Inc.: New York, 2000, pp 14.1.1–14.1.8.
Acknowledgements
This work was supported by grants from the Biomedical Research Council, National Medical Research Council and National Cancer Centre, all in Singapore. We thank Adrian Khoo for useful discussions on hepatocyte preparation, Serene Lok for technical assistance with hepatocyte implantations, Elsie Kok, Magdalene Koh and Leong Siew Hong for assistance with immunohistochemistry and image capture, Patricia Netto for help with electron microscopy and Dr Li Huihua for statistical guidance.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chen, N., Sivalingam, J., Tan, S. et al. Plasmid-electroporated primary hepatocytes acquire quasi-physiological secretion of human insulin and restore euglycemia in diabetic mice. Gene Ther 12, 655–667 (2005). https://doi.org/10.1038/sj.gt.3302446
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302446
Keywords
This article is cited by
-
Ex Vivo Gene Delivery to Hepatocytes: Techniques, Challenges, and Underlying Mechanisms
Annals of Biomedical Engineering (2012)
-
Insulin expressed from endogenously active glucose-responsive EGR1 promoter in bone marrow mesenchymal stromal cells as diabetes therapy
Gene Therapy (2010)
-
Treatment of diabetes by transplantation of drug-inducible insulin-producing gut cells
Journal of Molecular Medicine (2009)