Abstract
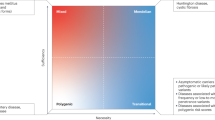
Rheumatoid arthritis (RA) is a chronic and potentially debilitating autoimmune disease. While novel therapies have emerged in recent years, disease remission is rarely achieved. RA is a complex trait, and the identifying of its susceptibility and severity genes has been anticipated to generate new targets for therapeutic intervention. However, finding those genes and understanding their function has been a challenging task. Studies in rodent intercrosses and congenics generated from inbred strains have been an important complementary strategy to identify arthritis genes, and understand how they operate to regulate disease. Furthermore, these new rodent arthritis genes will be new targets for therapeutic interventions, and will identify new candidate genes or candidate pathways for association studies in RA. In this review-opinion article I discuss RA genetics, difficulties involved in gene identification, and how rodent models can facilitate (1) the discovery of both arthritis susceptibility and severity genes, (2) studies of gene–environment interactions, (3) studies of gene–gender interactions, (4) epistasis, (5) functional characterization of the specific genes, (6) development of novel therapies and (7) how the information generated from rodent studies will be useful to understanding and potentially treating RA.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 digital issues and online access to articles
$119.00 per year
only $19.83 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Wilder R, Remmers E, Kawahito Y, Gulko P, Cannon G, Griffiths M . Genetic factors regulating experimental arthritis in mice and rats. In: Theophilopoulos A (ed). Current Directions in Autoimmunity. Karger: Basel, 1999, pp 121–165.
Holmdahl R, Lorentzen JC, Lu S, Olofsson P, Wester L, Holmberg J et al. Arthritis induced in rats with nonimmunogenic adjuvants as models for rheumatoid arthritis. Immunol Rev 2001; 184: 184–202.
Joe B . Quest for arthritis-causative genetic factors in the rat. Physiol Genomics 2006; 27: 1–11.
Seldin MF, Amos CI, Ward R, Gregersen PK . The genetics revolution and the assault on rheumatoid arthritis. Arthritis Rheum 1999; 42: 1071–1079.
Cornelis F, Faure S, Martinez M, Prud'homme JF, Fritz P, Dib C et al. New susceptibility locus for rheumatoid arthritis suggested by a genome-wide linkage study. Proc Natl Acad Sci USA 1998; 95: 10746–10750.
Jawaheer D, Seldin MF, Amos CI, Chen WV, Shigeta R, Monteiro J et al. A genomewide screen in multiplex rheumatoid arthritis families suggests genetic overlap with other autoimmune diseases. Am J Hum Genet 2001; 68: 927–936.
Jawaheer D, Seldin MF, Amos CI, Chen WV, Shigeta R, Etzel C et al. Screening the genome for rheumatoid arthritis susceptibility genes: a replication study and combined analysis of 512 multicase families. Arthritis Rheum 2003; 48: 906–916.
MacKay K, Eyre S, Myerscough A, Milicic A, Barton A, Laval S et al. Whole-genome linkage analysis of rheumatoid arthritis susceptibility loci in 252 affected sibling pairs in the United Kingdom. Arthritis Rheum 2002; 46: 632–639.
Shiozawa S, Hayashi S, Tsukamoto Y, Goko H, Kawasaki H, Wada T et al. Identification of the gene loci that predispose to rheumatoid arthritis. Int Immunol 1998; 10: 1891–1895.
Osorio y, Fortea J, Bukulmez H, Petit-Teixeira E, Michou L, Pierlot C et al. Dense genome-wide linkage analysis of rheumatoid arthritis, including covariates. Arthritis Rheum 2004; 50: 2757–2765.
Eyre S, Barton A, Shephard N, Hinks A, Brintnell W, MacKay K et al. Investigation of susceptibility loci identified in the UK rheumatoid arthritis whole-genome scan in a further series of 217 UK affected sibling pairs. Arthritis Rheum 2004; 50: 729–735.
John S, Shephard N, Liu G, Zeggini E, Cao M, Chen W et al. Whole-genome scan, in a complex disease, using 11 245 single-nucleotide polymorphisms: comparison with microsatellites. Am J Hum Genet 2004; 75: 54–64.
Amos CI, Chen WV, Lee A, Li W, Kern M, Lundsten R et al. High-density SNP analysis of 642 Caucasian families with rheumatoid arthritis identifies two new linkage regions on 11p12 and 2q33. Genes Immun 2006; 7: 277–286.
Remmers EF, Plenge RM, Lee AT, Graham RR, Hom G, Behrens TW et al. STAT4 and risk of rheumatoid arthritis and systemic lupus erythematosus. N Engl J Med 2007 (in press).
Plenge RM, Padyukov L, Remmers EF, Purcell S, Lee AT, Karlson EW et al. Replication of putative candidate-gene associations with rheumatoid arthritis in >4000 samples from North America and Sweden: association of susceptibility with PTPN22, CTLA4, and PADI4. Am J Hum Genet 2005; 77: 1044–1060.
Begovich AB, Carlton VE, Honigberg LA, Schrodi SJ, Chokkalingam AP, Alexander HC et al. A missense single-nucleotide polymorphism in a gene encoding a protein tyrosine phosphatase (PTPN22) is associated with rheumatoid arthritis. Am J Hum Genet 2004; 75: 330–337.
Suzuki A, Yamada R, Chang X, Tokuhiro S, Sawada T, Suzuki M et al. Functional haplotypes of PADI4, encoding citrullinating enzyme peptidylarginine deiminase 4, are associated with rheumatoid arthritis. Nat Genet 2003; 34: 395–402.
Okamoto K, Makino S, Yoshikawa Y, Takaki A, Nagatsuka Y, Ota M et al. Identification of I kappa BL as the second major histocompatibility complex-linked susceptibility locus for rheumatoid arthritis. Am J Hum Genet 2003; 72: 303–312.
Tokuhiro S, Yamada R, Chang X, Suzuki A, Kochi Y, Sawada T et al. An intronic SNP in a RUNX1 binding site of SLC22A4, encoding an organic cation transporter, is associated with rheumatoid arthritis. Nat Genet 2003; 35: 341–348.
Lander E, Kruglyak L . Genetic dissection of complex traits: guidelines for interpreting and reporting linkage results. Nat Genet 1995; 11: 241–247.
McKeigue PM . Prospects for admixture mapping of complex traits. Am J Hum Genet 2005; 76: 1–7.
Seldin MF, Shigeta R, Villoslada P, Selmi C, Tuomilehto J, Silva G et al. European population substructure: clustering of northern and southern populations. PLoS Genet 2006; 2: e143.
Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science 2006; 314: 1461–1463.
The Wellcome Trust Case Control Consortium. Genome-wide association study of 14 000 cases of seven common diseases and 3000 shared controls. Nature 2007; 447: 661–678.
Mikuls TR, Cerhan JR, Criswell LA, Merlino L, Mudano AS, Burma M et al. Coffee, tea, and caffeine consumption and risk of rheumatoid arthritis: results from the Iowa Women's Health Study. Arthritis Rheum 2002; 46: 83–91.
Silman AJ, Newman J, MacGregor AJ . Cigarette smoking increases the risk of rheumatoid arthritis. Results from a nationwide study of disease-discordant twins. Arthritis Rheum 1996; 39: 732–735.
Padyukov L, Silva C, Stolt P, Alfredsson L, Klareskog L . A gene-environment interaction between smoking and shared epitope genes in HLA-DR provides a high risk of seropositive rheumatoid arthritis. Arthritis Rheum 2004; 50: 3085–3092.
Sverdrup B, Kallberg H, Bengtsson C, Lundberg I, Padyukov L, Alfredsson L et al. Association between occupational exposure to mineral oil and rheumatoid arthritis: results from the Swedish EIRA case-control study. Arthritis Res Ther 2005; 7: R1296–R1303.
Ji H, Gauguier D, Ohmura K, Gonzalez A, Duchatelle V, Danoy P et al. Genetic influences on the end-stage effector phase of arthritis. J Exp Med 2001; 194: 321–330.
Brenner M, Meng HC, Yarlett NC, Griffiths MM, Remmers EF, Wilder RL et al. The non-major histocompatibility complex quantitative trait locus Cia10 contains a major arthritis gene and regulates disease severity, pannus formation, and joint damage. Arthritis Rheum 2005; 52: 322–332.
Brenner M, Meng HC, Yarlett NC, Joe B, Griffiths MM, Remmers EF et al. The non-MHC quantitative trait locus Cia5 contains three major arthritis genes that differentially regulate disease severity, pannus formation, and joint damage in collagen- and pristane-induced arthritis. J Immunol 2005; 174: 7894–7903.
Adarichev VA, Valdez JC, Bardos T, Finnegan A, Mikecz K, Glant TT . Combined autoimmune models of arthritis reveal shared and independent qualitative (binary) and quantitative trait loci. J Immunol 2003; 170: 2283–2292.
Adarichev VA, Nesterovitch AB, Bardos T, Biesczat D, Chandrasekaran R, Vermes C et al. Sex effect on clinical and immunologic quantitative trait loci in a murine model of rheumatoid arthritis. Arthritis Rheum 2003; 48: 1708–1720.
Otto JM, Chandrasekeran R, Vermes C, Mikecz K, Finnegan A, Rickert SE et al. A genome scan using a novel genetic cross identifies new susceptibility loci and traits in a mouse model of rheumatoid arthritis. J Immunol 2000; 165: 5278–5286.
Otto JM, Cs-Szabo G, Gallagher J, Velins S, Mikecz K, Buzas EI et al. Identification of multiple loci linked to inflammation and autoantibody production by a genome scan of a murine model of rheumatoid arthritis. Arthritis Rheum 1999; 42: 2524–2531.
Lorentzen JC, Glaser A, Jacobsson L, Galli J, Fakhrai-rad H, Klareskog L et al. Identification of rat susceptibility loci for adjuvant-oil-induced arthritis. Proc Natl Acad Sci USA 1998; 95: 6383–6387.
Vingsbo-Lundberg C, Nordquist N, Olofsson P, Sundvall M, Saxne T, Pettersson U et al. Genetic control of arthritis onset, severity and chronicity in a model for rheumatoid arthritis in rats. Nat Genet 1998; 20: 401–404.
Jirholt J, Cook A, Emahazion T, Sundvall M, Jansson L, Nordquist N et al. Genetic linkage analysis of collagen-induced arthritis in the mouse. Eur J Immunol 1998; 28: 3321–3328.
Yang HT, Jirholt J, Svensson L, Sundvall M, Jansson L, Pettersson U et al. Identification of genes controlling collagen-induced arthritis in mice: striking homology with susceptibility loci previously identified in the rat. J Immunol 1999; 163: 2916–2921.
Remmers EF, Longman RE, Du Y, O'Hare A, Cannon GW, Griffiths MM et al. A genome scan localizes five non-MHC loci controlling collagen-induced arthritis in rats. Nat Genet 1996; 14: 82–85.
Gulko PS, Kawahito Y, Remmers EF, Reese VR, Wang J, Dracheva SV et al. Identification of a new non-major histocompatibility complex genetic locus on chromosome 2 that controls disease severity in collagen- induced arthritis in rats. Arthritis Rheum 1998; 41: 2122–2131.
Kawahito Y, Cannon G, Gulko P, Remmers E, Longman R, Reese V et al. Localization of quantitative trait loci regulating adjuvant induced arthritis in rats: evidence for genetic factors common to multiple autoimmune diseases. J Immunol 1998; 161: 4411–4419.
Dracheva SV, Remmers EF, Gulko PS, Kawahito Y, Longman RE, Reese VR et al. Identification of a new quantitative trait locus on Chromosome 7 controlling disease severity of collagen-induced arthritis in rats. Immunogenetics 1999; 49: 787–791.
Furuya T, Salstrom JL, McCall-Vining S, Cannon GW, Joe B, Remmers EF et al. Genetic dissection of a rat model for rheumatoid arthritis: significant gender influences on autosomal modifier loci. Hum Mol Genet 2000; 9: 2241–2250.
Griffiths MM, Wang J, Joe B, Dracheva S, Kawahito Y, Shepard JS et al. Identification of four new quantitative trait loci regulating arthritis severity and one new quantitative trait locus regulating autoantibody production in rats with collagen-induced arthritis. Arthritis Rheum 2000; 43: 1278–1289.
Sokka T, Willoughby J, Yazici Y, Pincus T . Databases of patients with early rheumatoid arthritis in the USA. Clin Exp Rheumatol 2003; 21 (Suppl 31): S146–S153.
van der Helm-van Mil AH, le Cessie S, van Dongen H, Breedveld FC, Toes RE, Huizinga TW . A prediction rule for disease outcome in patients with recent-onset undifferentiated arthritis: how to guide individual treatment decisions. Arthritis Rheum 2007; 56: 433–440.
Sakaguchi N, Takahashi T, Hata H, Nomura T, Tagami T, Yamazaki S et al. Altered thymic T-cell selection due to a mutation of the ZAP-70 gene causes autoimmune arthritis in mice. Nature 2003; 426: 454–460.
Yu P, Constien R, Dear N, Katan M, Hanke P, Bunney TD et al. Autoimmunity and inflammation due to a gain-of-function mutation in phospholipase C gamma 2 that specifically increases external Ca2+ entry. Immunity 2005; 22: 451–465.
Atsumi T, Ishihara K, Kamimura D, Ikushima H, Ohtani T, Hirota S et al. A point mutation of Tyr-759 in interleukin 6 family cytokine receptor subunit gp130 causes autoimmune arthritis. J Exp Med 2002; 196: 979–990.
Horai R, Saijo S, Tanioka H, Nakae S, Sudo K, Okahara A et al. Development of chronic inflammatory arthropathy resembling rheumatoid arthritis in interleukin 1 receptor antagonist-deficient mice. J Exp Med 2000; 191: 313–320.
Keffer J, Probert L, Cazlaris H, Georgopoulos S, Kaslaris E, Kioussis D et al. Transgenic mice expressing human tumour necrosis factor: a predictive genetic model of arthritis. EMBO J 1991; 10: 4025–4031.
Holm BC, Xu HW, Jacobsson L, Larsson A, Luthman H, Lorentzen JC . Rats made congenic for Oia3 on chromosome 10 become susceptible to squalene-induced arthritis. Hum Mol Genet 2001; 10: 565–572.
Ribbhammar U, Flornes L, Backdahl L, Luthman H, Fossum S, Lorentzen JC . High resolution mapping of an arthritis susceptibility locus on rat chromosome 4, and characterization of regulated phenotypes. Hum Mol Genet 2003; 12: 2087–2096.
Brenner M, Laragione T, Mello A, Gulko PS . Cia25 on rat chromosome 12 regulates severity of autoimmune arthritis induced with pristane and with collagen. Ann Rheum Dis 2007; 66: 952–957.
Brenner M, Laragione T, Yarlett NC, Li W, Mello A, Gulko PS . Cia27 is a novel non-MHC arthritis severity locus on rat chromosome 10 syntenic to the rheumatoid arthritis 17q22–q25 locus. Genes Immun 2006; 7: 335–341.
Remmers EF, Joe B, Griffiths MM, Dobbins DE, Dracheva SV, Hashiramoto A et al. Modulation of multiple experimental arthritis models by collagen-induced arthritis quantitative trait loci isolated in congenic rat lines: different effects of non-major histocompatibility complex quantitative trait loci in males and females. Arthritis Rheum 2002; 46: 2225–2234.
Wester L, Olofsson P, Ibrahim SM, Holmdahl R . Chronicity of pristane-induced arthritis in rats is controlled by genes on chromosome 14. J Autoimmun 2003; 21: 305–313.
Olofsson P, Holmberg J, Tordsson J, Lu S, Akerstrom B, Holmdahl R . Positional identification of Ncf1 as a gene that regulates arthritis severity in rats. Nat Genet 2003; 33: 25–32.
Olofsson P, Nerstedt A, Hultqvist M, Nilsson EC, Andersson S, Bergelin A et al. Arthritis suppression by NADPH activation operates through an interferon-beta pathway. BMC Biol 2007; 5: 19.
Chanock SJ, Roesler J, Zhan S, Hopkins P, Lee P, Barrett DT et al. Genomic structure of the human p47-phox (NCF1) gene. Blood Cells Mol Dis 2000; 26: 37–46.
Vazquez N, Lehrnbecher T, Chen R, Christensen BL, Gallin JI, Malech H et al. Mutational analysis of patients with p47-phox-deficient chronic granulomatous disease: the significance of recombination events between the p47-phox gene (NCF1) and its highly homologous pseudogenes. Exp Hematol 2001; 29: 234–243.
Brenner M, Braun C, Oster M, Gulko PS . Thermal signature analysis as a novel method for evaluating inflammatory arthritis activity. Ann Rheum Dis 2006; 65: 306–311.
Caulfield JP, Hein A, Dynesius-Trentham R, Trentham DE . Morphologic demonstration of two stages in the development of type II collagen-induced arthritis. Lab Invest 1982; 46: 321–343.
Pando JA, Duray P, Yarboro C, Gourley MF, Klippel JH, Schumacher HR . Synovitis occurs in some clinically normal and asymptomatic joints in patients with early arthritis. J Rheumatol 2000; 27: 1848–1854.
Kraan MC, Versendaal H, Jonker M, Bresnihan B, Post WJ, t Hart BA et al. Asymptomatic synovitis precedes clinically manifest arthritis. Arthritis Rheum 1998; 41: 1481–1488.
Laragione T, Brenner M, Yarlett NC, Mello A, Miller EJ, Metz CN et al. The arthritis severity quantitative trait locus Cia7 regulates neutrophil migration into inflammatory sites. Genes Immun 2007; 8: 147–153.
Laragione T, Yarlett NC, Brenner M, Mello A, Sherry B, Miller EJ et al. The arthritis severity quantitative trait loci Cia4 and Cia6 regulate neutrophil migration into inflammatory sites and levels of TNF-alpha and nitric oxide. J Immunol 2007; 178: 2344–2351.
Brenner M, Laragione T, Yarlett NC, Gulko PS . Genetic regulation of T regulatory, CD4, and CD8 cell numbers by the arthritis severity loci Cia5a, Cia5d and the MHC in the rat. Mol Med 2007; 13: 277–287.
Yu X, Bauer K, Wernhoff P, Ibrahim SM . Using an advanced intercross line to identify quantitative trait loci controlling immune response during collagen-induced arthritis. Genes Immun 2007; 8: 296–301.
Yu X, Bauer K, Wernhoff P, Koczan D, Moller S, Thiesen HJ et al. Fine mapping of collagen-induced arthritis quantitative trait loci in an advanced intercross line. J Immunol 2006; 177: 7042–7049.
Johannesson M, Karlsson J, Wernhoff P, Nandakumar KS, Lindqvist AK, Olsson L et al. Identification of epistasis through a partial advanced intercross reveals three arthritis loci within the Cia5 QTL in mice. Genes Immun 2005; 6: 175–185.
Lindqvist AK, Johannesson M, Johansson AC, Nandakumar KS, Blom AM, Holmdahl R . Backcross and partial advanced intercross analysis of nonobese diabetic gene-mediated effects on collagen-induced arthritis reveals an interactive effect by two major loci. J Immunol 2006; 177: 3952–3959.
Cordell HJ . Epistasis: what it means, what it doesn't mean, and statistical methods to detect it in humans. Hum Mol Genet 2002; 11: 2463–2468.
John S, Amos C, Shephard N, Chen W, Butterworth A, Etzel C et al. Linkage analysis of rheumatoid arthritis in US and UK families reveals interactions between HLA-DRB1 and loci on chromosomes 6q and 16p. Arthritis Rheum 2006; 54: 1482–1490.
Kochi Y, Yamada R, Suzuki A, Harley JB, Shirasawa S, Sawada T et al. A functional variant in FCRL3, encoding Fc receptor-like 3, is associated with rheumatoid arthritis and several autoimmunities. Nat Genet 2005; 37: 478–485.
Yazdani-Biuki B, Brickmann K, Wohlfahrt K, Mueller T, Marz W, Renner W et al. The MHC2TA -168A>G gene polymorphism is not associated with rheumatoid arthritis in Austrian patients. Arthritis Res Ther 2006; 8: R97.
Eyre S, Bowes J, Potter C, Worthington J, Barton A . Association of the FCRL3 gene with rheumatoid arthritis: a further example of population specificity? Arthritis Res Ther 2006; 8: R117.
Newman WG, Zhang Q, Liu X, Walker E, Ternan H, Owen J et al. Rheumatoid arthritis association with the FCRL3 -169C polymorphism is restricted to PTPN22 1858T-homozygous individuals in a Canadian population. Arthritis Rheum 2006; 54: 3820–3827.
Meng HC, Griffiths MM, Remmers EF, Kawahito Y, Li W, Neisa R et al. Identification of two novel female-specific non-major histocompatibility complex loci regulating collagen-induced arthritis severity and chronicity, and evidence of epistasis. Arthritis Rheum 2004; 50: 2695–2705.
Ahlqvist E, Bockermann R, Holmdahl R . Fragmentation of two quantitative trait loci controlling collagen-induced arthritis reveals a new set of interacting subloci. J Immunol 2007; 178: 3084–3090.
Olofsson P, Wernhoff P, Holmberg J, Holmdahl R . Two-loci interaction confirms arthritis-regulating quantitative trait locus on rat chromosome 6. Genomics 2003; 82: 652–659.
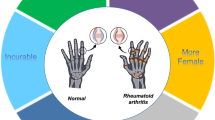
Weyand CM, Schmidt D, Wagner U, Goronzy JJ . The influence of sex on the phenotype of rheumatoid arthritis. Arthritis Rheum 1998; 41: 817–822.
Fex E, Jonsson K, Johnson U, Eberhardt K . Development of radiographic damage during the first 5–6 year of rheumatoid arthritis. A prospective follow-up study of a Swedish cohort. Br J Rheumatol 1996; 35: 1106–1115.
Yamada R, Tanaka T, Unoki M, Nagai T, Sawada T, Ohnishi Y et al. Association between a single-nucleotide polymorphism in the promoter of the human interleukin-3 gene and rheumatoid arthritis in Japanese patients, and maximum-likelihood estimation of combinatorial effect that two genetic loci have on susceptibility to the disease. Am J Hum Genet 2001; 68: 674–685.
Jansson L, Holmdahl R . The Y chromosome-linked ‘autoimmune accelerating’ yaa gene suppresses collagen-induced arthritis. Eur J Immunol 1994; 24: 1213–1217.
Subramanian S, Tus K, Li QZ, Wang A, Tian XH, Zhou J et al. A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. Proc Natl Acad Sci USA 2006; 103: 9970–9975.
Weinblatt ME, Kremer JM, Bankhurst AD, Bulpitt KJ, Fleischmann RM, Fox RI et al. A trial of etanercept, a recombinant tumor necrosis factor receptor:Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med 1999; 340: 253–259.
Lipsky PE, van der Heijde DM, St Clair EW, Furst DE, Breedveld FC, Kalden JR et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-tumor necrosis factor trial in rheumatoid arthritis with Concomitant Therapy Study Group. N Engl J Med 2000; 343: 1594–1602.
Weinblatt ME, Keystone EC, Furst DE, Kavanaugh AF, Chartash EK, Segurado OG . Long-term efficacy and safety of adalimumab plus methotrexate in patients with rheumatoid arthritis: ARMADA 4-year extended study. Ann Rheum Dis 2006; 65: 753–759.
Cohen SB, Woolley JM, Chan W . Interleukin 1 receptor antagonist anakinra improves functional status in patients with rheumatoid arthritis. J Rheumatol 2003; 30: 225–231.
Choy EH, Isenberg DA, Garrood T, Farrow S, Ioannou Y, Bird H et al. Therapeutic benefit of blocking interleukin-6 activity with an anti-interleukin-6 receptor monoclonal antibody in rheumatoid arthritis: a randomized, double-blind, placebo-controlled, dose-escalation trial. Arthritis Rheum 2002; 46: 3143–3150.
Kremer JM, Westhovens R, Leon M, Di Giorgio E, Alten R, Steinfeld S et al. Treatment of rheumatoid arthritis by selective inhibition of T-cell activation with fusion protein CTLA4Ig. N Engl J Med 2003; 349: 1907–1915.
Edwards JC, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close DR et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med 2004; 350: 2572–2581.
Becker KG, Simon RM, Bailey-Wilson JE, Freidlin B, Biddison WE, McFarland HF et al. Clustering of non-major histocompatibility complex susceptibility candidate loci in human autoimmune diseases. Proc Natl Acad Sci USA 1998; 95: 9979–9984.
Johansson CM, Zunec R, Garcia MA, Scherbarth HR, Tate GA, Paira S et al. Chromosome 17p12-q11 harbors susceptibility loci for systemic lupus erythematosus. Hum Genet 2004; 115: 230–238.
Moser KL, Neas BR, Salmon JE, Yu H, Gray-McGuire C, Asundi N et al. Genome scan of human systemic lupus erythematosus: evidence for linkage on chromosome 1q in African-American pedigrees. Proc Natl Acad Sci USA 1998; 95: 14869–14874.
Lindqvist AK, Steinsson K, Johanneson B, Kristjansdottir H, Arnasson A, Grondal G et al. A susceptibility locus for human systemic lupus erythematosus (hSLE1) on chromosome 2q. J Autoimmun 2000; 14: 169–178.
Lee YA, Ruschendorf F, Windemuth C, Schmitt-Egenolf M, Stadelmann A, Nurnberg G et al. Genomewide scan in german families reveals evidence for a novel psoriasis-susceptibility locus on chromosome 19p13. Am J Hum Genet 2000; 67: 1020–1024.
Laval SH, Timms A, Edwards S, Bradbury L, Brophy S, Milicic A et al. Whole-genome screening in ankylosing spondylitis: evidence of non-MHC genetic-susceptibility loci. Am J Hum Genet 2001; 68: 918–926.
Haines JL, Bradford Y, Garcia ME, Reed AD, Neumeister E, Pericak-Vance MA et al. Multiple susceptibility loci for multiple sclerosis. Hum Mol Genet 2002; 11: 2251–2256.
Pericak-Vance MA, Rimmler JB, Martin ER, Haines JL, Garcia ME, Oksenberg JR et al. Linkage and association analysis of chromosome 19q13 in multiple sclerosis. Neurogenetics 2001; 3: 195–201.
van Heel DA, Fisher SA, Kirby A, Daly MJ, Rioux JD, Lewis CM . Inflammatory bowel disease susceptibility loci defined by genome scan meta-analysis of 1952 affected relative pairs. Hum Mol Genet 2004; 13: 763–770.
Rioux JD, Silverberg MS, Daly MJ, Steinhart AH, McLeod RS, Griffiths AM et al. Genomewide search in Canadian families with inflammatory bowel disease reveals two novel susceptibility loci. Am J Hum Genet 2000; 66: 1863–1870.
Haines JL, Ter-Minassian M, Bazyk A, Gusella JF, Kim DJ, Terwedow H et al. A complete genomic screen for multiple sclerosis underscores a role for the major histocompatability complex. The Multiple Sclerosis Genetics Group [see comments]. Nat Genet 1996; 13: 469–471.
Satsangi J, Parkes M, Louis E, Hashimoto L, Kato N, Welsh K et al. Two stage genome-wide search in inflammatory bowel disease provides evidence for susceptibility loci on chromosomes 3, 7 and 12. Nat Genet 1996; 14: 199–202.
Enlund F, Samuelsson L, Enerback C, Inerot A, Wahlstrom J, Yhr M et al. Analysis of three suggested psoriasis susceptibility loci in a large Swedish set of families: confirmation of linkage to chromosome 6p (HLA region), and to 17q, but not to 4q. Hum Hered 1999; 49: 2–8.
Samuelsson L, Enlund F, Torinsson A, Yhr M, Inerot A, Enerback C et al. A genome-wide search for genes predisposing to familial psoriasis by using a stratification approach. Hum Genet 1999; 105: 523–529.
Sawcer S, Jones HB, Feakes R, Gray J, Smaldon N, Chataway J et al. A genome screen in multiple sclerosis reveals susceptibility loci on chromosome 6p21 and 17q22. Nat Genet 1996; 13: 464–468.
Vitale E, Cook S, Sun R, Specchia C, Subramanian K, Rocchi M et al. Linkage analysis conditional on HLA status in a large North American pedigree supports the presence of a multiple sclerosis susceptibility locus on chromosome 12p12. Hum Mol Genet 2002; 11: 295–300.
Rioux JD, Xavier RJ, Taylor KD, Silverberg MS, Goyette P, Huett A et al. Genome-wide association study identifies new susceptibility loci for Crohn disease and implicates autophagy in disease pathogenesis. Nat Genet 2007; 39: 596–604.
Sale MM, FitzGerald LM, Charlesworth JC, Bowden DW, Rich SS . Evidence for a novel type 1 diabetes susceptibility locus on chromosome 8. Diabetes 2002; 51 (Suppl 3): S316–S319.
Davies JL, Kawaguchi Y, Bennett ST, Copeman JB, Cordell HJ, Pritchard LE et al. A genome-wide search for human type 1 diabetes susceptibility genes. Nature 1994; 371: 130–136.
Barton A, Eyre S, Myerscough A, Brintnell B, Ward D, Ollier WE et al. High resolution linkage and association mapping identifies a novel rheumatoid arthritis susceptibility locus homologous to one linked to two rat models of inflammatory arthritis. Hum Mol Genet 2001; 10: 1901–1906.
Saarela J, Schoenberg Fejzo M, Chen D, Finnila S, Parkkonen M, Kuokkanen S et al. Fine mapping of a multiple sclerosis locus to 2.5 Mb on chromosome 17q22–q24. Hum Mol Genet 2002; 11: 2257–2267.
Tomfohrde J, Silverman A, Barnes R, Fernandez-Vina MA, Young M, Lory D et al. Gene for familial psoriasis susceptibility mapped to the distal end of human chromosome 17q. Science 1994; 264: 1141–1145.
Nair RP, Henseler T, Jenisch S, Stuart P, Bichakjian CK, Lenk W et al. Evidence for two psoriasis susceptibility loci (HLA and 17q) and two novel candidate regions (16q and 20p) by genome-wide scan. Hum Mol Genet 1997; 6: 1349–1356.
Ebers GC, Kukay K, Bulman DE, Sadovnick AD, Rice G, Anderson C et al. A full genome search in multiple sclerosis. Nat Genet 1996; 13: 472–476.
Dyment DA, Willer CJ, Scott B, Armstrong H, Ligers A, Hillert J et al. Genetic susceptibility to MS: a second stage analysis in Canadian MS families. Neurogenetics 2001; 3: 145–151.
Paterson AD, Petronis A . Sex of affected sibpairs and genetic linkage to type 1 diabetes. Am J Med Genet 1999; 84: 15–19.
Mein CA, Esposito L, Dunn MG, Johnson GC, Timms AE, Goy JV et al. A search for type 1 diabetes susceptibility genes in families from the United Kingdom. Nat Genet 1998; 19: 297–300.
Michou L, Lasbleiz S, Rat AC, Migliorini P, Balsa A, Westhovens R et al. Linkage proof for PTPN22, a rheumatoid arthritis susceptibility gene and a human autoimmunity gene. Proc Natl Acad Sci USA 2007; 104: 1649–1654.
Veal CD, Clough RL, Barber RC, Mason S, Tillman D, Ferry B et al. Identification of a novel psoriasis susceptibility locus at 1p and evidence of epistasis between PSORS1 and candidate loci. J Med Genet 2001; 38: 7–13.
Reich D, Patterson N, De Jager PL, McDonald GJ, Waliszewska A, Tandon A et al. A whole-genome admixture scan finds a candidate locus for multiple sclerosis susceptibility. Nat Genet 2005; 37: 1113–1118.
Acknowledgements
Funded by National Institutes of Health Grants R01-AR46213, R01-AR052439 (NIAMS) and R01-AI54348 (NIAID).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gulko, P. Contribution of genetic studies in rodent models of autoimmune arthritis to understanding and treatment of rheumatoid arthritis. Genes Immun 8, 523–531 (2007). https://doi.org/10.1038/sj.gene.6364419
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gene.6364419