Abstract
Scope and purpose
This guideline aimed to provide recommendations for the diagnosis of bisphosphonate-associated osteonecrosis of the jaw in both the oncology and osteoporosis patient populations, for dental and medical practitioners including dentists, oral surgeons, oral pathologists, general practitioners and internal medicine specialists. The recommendations are intended to address both prevention and treatment strategies.
Methods
A consensus-based guideline was developed by a multidisciplinary task force including representatives from national and international societies representing the disciplines of oral surgery, dentistry, oral pathology, oral medicine, endocrinology, rheumatology and oncology. The task force reviewed data collected for a systematic review and prepared discussion papers. The systematic review included searches of Medline, Embase and a manual search of the bibliographies of published articles. A draft guideline was circulated to all members of the task force as well as external experts, and their feedback incorporated into the final document.
Recommendations
The main recommendations are summarised in Table 1. The task force also recommended that a registry be maintained for all identified cases.
Similar content being viewed by others
Commentary
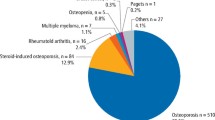
Bisphosphonates are mainly used for the treatment of osteoporosis, but they are also used in the treatment of cancer. Cancer patients often take them in higher doses than are used for noncancer treatments, and often do so intravenously. Bisphosphonate-associated osteonecrosis is a rare clinical entity that is poorly understood: estimates of its incidence in patients on oral bisphosphonate therapy range between 1:10 000 and 1:100 000, rising to 1:10–1:100 in cancer patients taking high-dose intravenous bisphosphonates.1 The number of cases is increasing, however: this may be caused by improving recognition of the condition, the use of more potent bisphosphonates, or the increased use of this group of drugs. Osteonecrosis is the jaw (ONJ) is diagnosed clinically as the presence of exposed bone in the maxillofacial region for more than 8 weeks in the absence of radiotherapy to the jaw.2
As well as more cases, the number of publications related to ONJ has also gone up. A simple search of Medline (search terms: bisphosphonates and osteonecrosis) identifies over 750 papers with about 140 reviews. As found in the review upon which the guideline is based, the amount of high-quality information on this topic is currently limited, however.
The recommendations from the Canadian task group are in line with those from other groups.3, 4 Reading other guidance documents reveals two main areas of disagreement: the use of prophylactic antibiotics prior to minor surgical procedures when people are taking bisphosphonates, and the discontinuation of bisphosphonate treatment (should the patient's clinical situation allow) for the treatment of ONJ.
Regarding prophylactic antibiotics, the American Dental Association (ADA) expert panel3 found no evidence that their use was effective in preventing ONJ and recommended that, “Prophylactic antibiotics after a surgical procedure should be based on the risk of an infection and NOT because the patient is taking a bisphosphonate.” The British Dental Association (BDA) factfile on bisphosphonates4 takes a similar position.
The interruption of bisphosphonate therapy for 3–6 months is recommended in this Canadian guideline for nonemergency invasive dental treatment, but the half-life of bisphosphonates in the skeleton is high and there is only anecdotal evidence to support this approach. The guideline does highlight that cessation of bisphosphonate therapy for several months does not seem to have a detrimental effect on osteoporosis management.5 In view of the lack of robust evidence, the BDA factfile's recommendation, to assess the clinical situation and discuss it with the patient's physician or oncologist before stopping the bisphosphonates, seems the more realistic approach.
The guidance identifies three stages of ONJ (see Table 2) and a range of treatments for each stage. No prospective studies assessing the effectiveness of these treatments were identified, so it is recommended that conservative approaches are the most effective. This remains a relatively rare condition despite the increasing numbers, but is one of which dentists should be aware. These broad consensus guidelines provide useful advice to practitioners. The ADA and BDA recommendations (Table 3 and see www.bda.org) provide additional specific advice for dentists.
The available evidence-base for ONJ is limited at present as this is a relatively new clinical entity, with the first cases being reported in 2003.6 There are knowledge gaps, therefore, as highlighted in this guideline. These include a lack of understanding of the pathogenesis and true incidence of ONJ, and the prospective data needed to stratify risk factors and develop prevention and management recommendations. These well-developed multidisciplinary guidelines are a useful step to raising awareness of the profession's role in prevention and management of this condition.
References
Arrain Y, Masud T . Recent recommendations on bisphophonate associated osteonecrosis of the jaw. Dental Update 2008; 35:238–242
Barker K, Rogers S . Bisphosphate associated osteonecrosis of the jaws: a guide for the general practitioner. Dental Update 2006; 33:270–275.
American Dental Association. Dental Management of Patients Receiving Oral Bisphosphonate Therapy. Expert Panel Recommendations Report of the Council on Scientific Affairs. Chicago: American Dental Association; 2008.
British Dental Association. Bisphosphonates. Fact File. London: British Dental Association; 2008.
Black DM, Schwartz AV, Ensrud KE, et al. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. J Am Med Assoc 2006; 296:2927–2938.
Marx RE . Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg 2003; 61:1115–1117.
Author information
Authors and Affiliations
Additional information
Address for correspondence: A.A. Khan, Professor of Clinical Medicine, Divisions of Endocrinology and Geriatrics, McMaster University, Hamilton, Ontario, Canada
Khan AA, Sándor GK, Dore E, et al. Canadian consensus practice guidelines for bisphosphonate associated osteonecrosis of the jaw. J Rheumatol 2008; 35:1391–1397
Rights and permissions
About this article
Cite this article
Richards, D. Guidelines for bisphosphonate-associated osteonecrosis of the jaw. Evid Based Dent 9, 101–102 (2008). https://doi.org/10.1038/sj.ebd.6400608
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ebd.6400608
This article is cited by
-
Relevance of surgical management of patients affected by bisphosphonate-associated osteonecrosis of the jaws. A prospective clinical and radiological study
Clinical Oral Investigations (2014)
-
Dental risk factors for osteonecrosis of the jaws: a CONDOR case?control study
Clinical Oral Investigations (2013)