Abstract
Study design:
Cross-sectional study comparing athletes with spinal cord injury (SCI) and age and body mass index matched able-bodied controls (AB).
Objective:
To examine the impact of exercise training on the relation between whole body, regional and intermuscular adipose tissue (IMAT) and glucose tolerance, insulin action and lipid profile.
Setting:
University Research Laboratory, USA.
Methods:
Fourteen college-aged athletes with SCI (seven men; duration of injury 16.5±5.7 years, level of injury T5-L5) and 17 sedentary AB (eight men) were assessed for body composition via dual-energy X-ray absorptiometry (DXA) and magnetic resonance imaging. Insulin sensitivity index (ISI) was determined via 2-h oral glucose challenge; standard lipid profile was determined from fasting blood samples.
Results:
Although ISI was 30% higher in SCI, there were no significant differences between groups in glucose and insulin responses or in lipid measures. Adjusting for absolute and relative thigh IMAT area, fasting insulin (13.8±5.3 μIU, 16.3±5.6 μIU; P<0.05; SCI vs AB respectively) and ISI (4.0±1.4, 3.1±1.3; P<0.05) were significantly better among SCI athletes compared to AB. Measures of adiposity did not correlate with glucose response or most lipid measures. Within SCI and AB, respectively, ISI correlated strongly (all P<0.05) with absolute (r=−0.70, −0.54) and relative IMAT (r=−0.54, −0.50), than with trunk (r=−0.62, −0.64) and whole body fat mass (r=−0.61, −0.64).
Conclusion:
Habitual physical activity can maintain insulin sensitivity in SCI compared to sedentary AB controls. Total body fat mass, central adiposity and thigh IMAT appear to impact risk for metabolic disease in SCI individuals with IMAT playing a larger role in SCI than AB.
Similar content being viewed by others
Introduction
Similar to other populations, the favorable impact of exercise training on body composition,1, 2, 3, 4, 5, 6 glucose and insulin levels7 and the lipid profile1, 2, 3, 4, 5, 8 has been investigated in the spinal cord injury (SCI) population. Beyond whole body measures, novel adipose tissue depots measured by magnetic resonance imaging (MRI) allows for the measurement of subcutaneous adipose tissue and intermuscular adipose tissue (IMAT).9 Greater adipose infiltration of the muscle in obese10 and elderly,11 and abdominal obesity are associated with glucose intolerance,12 insulin resistance13 and cardiovascular disease14 and IMAT has been associated with type II diabetes15 and metabolic syndrome.16 To date, no studies have utilized MRI to assess the impact of physical activity on the relation between adipose depots and risk for metabolic disease in the SCI population.
In this context, the aim of this study was to assess the effect of habitual physical activity on the association between body composition and glucose tolerance, insulin sensitivity and the lipid profile of individuals with SCI compared to sedentary able-bodied (AB) individuals. A major focus of this investigation was to examine the relation of metabolic indices with thigh and arm muscle and fat in paraplegics, with special focus on IMAT. For this study, IMAT is defined as adipose tissue located beneath the fascia lata and between muscles.9, 10
Methods
Subjects
Fourteen highly active athletes with SCI (seven men, seven women; duration of injury 16.5±5.7 years, level of injury T5-L5) were recruited from university varsity athletic teams and compared to 17 age- and body mass index (BMI)-matched sedentary AB reference controls (eight men, nine women). The SCI athletes participated in the varsity athletic program between 1 and 5 years, which involved 12 h of sport-specific and 3 h of resistance training per week, while the AB sedentary subjects participated in less than 60 min of purposeful physical activity per week throughout the past 6 months. All subjects signed a consent form approved by the university's Institutional Review Board upon enrollment in the study.
Anthropometry
Body weight was measured on a calibrated digital scale (Tanita, Model BWB-627A), with SCI subjects transferring onto a tare-weighted stool. Height was determined with all subjects in the supine position using a Gulick II retractable measuring tape (Country Technologies Inc., Gay Mills, WI, USA) from the top of the head to the bottom of the heel. SCI individuals that could not extend all joints were segmented by joint and measured accordingly. BMI was computed as weight in kg divided by height in m2 (kg/m2). Waist circumference measures were taken in the supine position at the level of the umbilicus.
Dual-energy X-ray absorptiometry
Whole body and regional composition was measured using dual-energy X-ray absorptiometry (DXA) (Hologic QDR 4500A, software version 11.1:3, Waltham, MA, USA). Precision for DXA measurements of interest is between 1 and 1.5% in our laboratory.
Magnetic resonance imaging
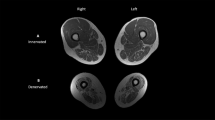
Images of the right thigh and right upper arm were collected on a 1.5 T magnet (GE Signa Echospeed, Milwaukee, WI, USA; repetition time, 100 ms; echo time, 4.2 ms; field of view, 40 cm; matrix 256 × 256). T1-weighted transaxial images, 0.5 cm thick and 0.25 cm apart, were taken from the upper arm and thigh using a whole body coil. Images used for analysis were centered at the midpoint of the limb of interest and included the mid-slice and one proximal and one distal slice with values averaged for data analysis. Midpoint of the upper arm was determined as half way between the acromion and olecranon processes. Midpoint of the thigh was determined as half way between the inguinal crease and proximal border of the patella. Analyses of the images were performed using Winvessel 2.011 software (Ron Meyer, PhD at Michigan State University). Images were automatically segmented into fat, skeletal muscle and background bone regions as described previously.16, 17, 18
To correct for differences in muscle area and limb size, IMAT areas of a given limb were divided by the muscle area of that limb, providing the relative IMAT for the limb. Similarly, the muscle areas and subcutaneous fat areas of a given limb were divided by the whole area of the respective limb, resulting in the relative muscle and subcutaneous fat areas for the limb. Two individuals analyzed the slices. Inter-rater reliability was excellent with correlation coefficients and absolute error (cm2) being 1.0, 0.30±0.36 (whole area), 1.0, 0.41±0.41 (muscle area), 0.99, 0.18±0.20 (IMAT area), and 1.0, 0.52±0.52 (subcutaneous fat area).
Oral glucose tolerance test
A 75-g, 2-h oral glucose tolerance test (OGTT) was conducted following a 12–14 h fast, at least 24 h following the last bout of exercise for SCI, whereas AB participated in no physical activity the day before the OGTT. Blood samples were drawn from an antecubital vein in the fasted state and 30, 60, 90 and 120 min after ingestion of the glucose beverage. Blood not immediately analyzed was stored at −80°C. Plasma glucose concentrations were measured by the glucose oxidase method using an automated glucose analyzer (YSI 2300, Yellow Springs, OH, USA) and plasma insulin concentrations were measured by a double-antibody radioimmunoassay (ImmuChem, Belgium). The functional sensitivity of the assay was determined to be 4.2 μIU/ml. The total areas under the glucose and insulin curves (area under the curve (AUC)) were calculated using the trapezoidal rule. The insulin sensitivity index of Matsuda and DeFronzo was calculated as (10 000/√ (fasting glucose × fasting insulin) × (mean OGTT glucose × mean OGTT insulin concentration). The Homeostasis Model Assessment of insulin resistance (HOMA) was calculated as ((fasting insulin × fasting glucose)/405).
Lipid profile
Fasting total cholesterol, high-density lipoprotein cholesterol (HDL-C) and triacylglycerol were determined by enzymatic assays (Infinity, Thermo Electron, Pittsburgh, PA, USA) as was non-esterified fatty acid (NEFA) (Wako Diagnostics, Richmond, VA, USA). Very low-density lipoprotein and low-density lipoprotein cholesterol (LDL-C) were calculated using the Friedewald equation.19 All intra- and inter-assay %CV were below 6.3 and 8.1%, respectively.
Statistical analyses
The statistical analyses were performed with SPSS 14.0.0. Data were assessed for normality, and absolute and relative arm and thigh IMAT, insulin, insulin AUC, HOMA, total cholesterol, LDL-C and NEFA were normalized by log transformation. Two-way analysis of variance (ANOVA; gender by group) and analysis of covariance (ANCOVA) and partial correlational analyses, controlling for various measures of adiposity, sex (because of sex differences in body composition) and duration of injury (SCI only) were used to explore differences between the groups. Statistical significance was accepted at P-value<0.05.
Results
The groups were matched on age and BMI and did not differ in percentage body fat (Table 1). The AB controls had higher fat mass, trunk fat mass, greater thigh muscle area and thigh muscle area relative to whole thigh area, and smaller thigh IMAT and thigh IMAT relative to muscle area (all P<0.01) than the SCI athletes. Alternatively, SCI athletes had greater arm muscle area and arm muscle area relative to whole arm area, and smaller arm IMAT relative to arm muscle area (all P<0.05) than the AB controls. Groups did not differ in arm IMAT area.
Although SCI athletes had lower insulin values, there were no significant differences between the groups in responses to the glucose challenge (Table 2) except in plasma glucose at 60 min during the OGTT (Figure 1). Using ANCOVA to control for total fat mass or central adiposity group differences did not become significant; however, controlling for either absolute or relative thigh IMAT area, all insulin sensitivity measures were significantly better among the SCI athletes compared to the AB controls (Figure 2). Adjusting for relative thigh muscle area had similar effects on insulin sensitivity; however, adjusting for absolute thigh muscle area or absolute or relative arm IMAT or muscle areas did not impact the group differences. Regarding lipids, no group differences existed except adjusting for relative thigh muscle area resulted in lower LDL-C among SCI athletes. Adjusting for total body fat mass, trunk fat mass, or waist circumference had little effects on lipids; only HDL-C levels became significantly higher among SCI athletes (P<0.01; data not shown).
Glucose (a) and insulin (b) responses to the oral glucose tolerance test. SCI, individuals with spinal cord injury, AB, able-bodied individuals. 1P<0.05 for group when corrected for thigh IMAT, 2P<0.05 for group when corrected for thigh IMAT/muscle, 3P<0.05 when corrected for thigh muscle/whole area, 4P<0.05 for group unadjusted. Values are means and s.e.
Insulin sensitivity as assessed by the insulin sensitivity index (a) and insulin area under the curve (b) after adjustment for thigh IMAT (absolute and relative) and thigh muscle (relative) areas. SCI, individuals with spinal cord injury, AB, able-bodied individuals, AUC, area under the curve, IMAT, intermuscular adipose tissue. 1P<0.05 for group. Values are estimated adjusted means and s.e.
The relative importance of whole body and regional fatness and ectopic adiposity on metabolic outcomes by group status was further explored by partial correlational analyses (Table 3). After adjusting for gender and duration of injury, whole body lean soft tissue was not correlated with metabolic outcomes in either group (P>0.05, data not shown). Subcutaneous thigh fat was a strong predictor of insulin response in both groups (r=0.53–0.71); however, after adjusting for whole body fat mass the relation diminished. No measures of adiposity were related to fasting or glucose AUC in either group. In AB controls, measures of total body fat mass and central adiposity were more strongly related to all measures of insulin than absolute or relative IMAT. In SCI athletes, the total body fat mass, estimates of central adiposity and IMAT were similarly related to insulin measures.
Among the AB controls, no adiposity measures were significantly correlated with lipid measures. Among the SCI athletes, measures of central adiposity were positively related to total cholesterol, LDL-C, and HDL-C, the latter an effect that is difficult to explain. Relative and absolute IMAT did not correlate significantly with any of the blood lipid measures in either group.
Discussion
The primary finding of this study is that habitual high-level physical activity can maintain metabolic outcomes in individuals with SCI to the same level as sedentary AB individuals despite the greatly reduced muscle mass activation. This is encouraging as metabolic disease (that is, type II diabetes mellitus) appears decades earlier in SCI individuals due to greatly reduced activity levels and increased risk for obesity.20 Moreover, indices of whole body and regional adiposity differ in their relation to insulin dynamics comparing young SCI athletes and AB controls. Our findings extend the recent literature regarding muscle fat and the interactive implications for adiposity, physical activity and metabolic disease risk.
It is evident in the literature that SCI individuals have increased risk for metabolic disorders compared to the AB population including impaired glucose tolerance and insulin resistance.21, 22 Little research has investigated the effect of physical activity on glucose tolerance and insulin action in the SCI population. Electrical stimulation of the lower-body muscles in paraplegics has been shown to improve glucose uptake in the paralyzed muscle23 as well as glucose tolerance24 and insulin sensitivity.23 However, the effect of upper-body exercise is not as clear. Some studies indicate that physical activity is not associated with fasting glucose,1, 4 glucose response to OGTT,1 fasting insulin4 or insulin response to OGTT4 in SCI individuals. Alternatively, high-intensity upper-body physical activity has been shown to improve insulin sensitivity.7 However, none of these studies included an AB comparison group.1, 4, 7 It is probable that the young age and high level of training of our subjects impacted our findings.
The use of MRI to measure body composition has been shown to be of particular relevance in assessing muscle quality and adipose tissue in the SCI population.17 In the AB population, there is a positive relation between thigh IMAT and risk for type II diabetes15 and metabolic syndrome16 and a negative relation between subcutaneous thigh fat and glucose levels25 and metabolic syndrome.16 Recent evidence suggests that thigh IMAT and subcutaneous fat areas are increased in sedentary SCI individuals and that relative IMAT is a strong predictor of plasma glucose suggesting an increased risk for impaired glucose tolerance and insulin resistance.18 Although thigh subcutaneous fat was a strong predictor of metabolic outcomes within group, this effect was largely due to the relation to whole body fat mass and notably the relation was in the positive direction (that is, not protective). Our findings regarding thigh IMAT suggest that insulin sensitivity is associated with thigh IMAT in SCI individuals and that high-volume upper-body physical activity can protect against the risk of insulin resistance resulting from thigh IMAT accumulation and loss of muscle in this population.
For prevention of metabolic disorders, an important question in the SCI population is whether an increase in adipose or loss of muscle tissue is the major contributor to metabolic disease risk. There are indications that both tissues play a role in metabolic disorders in the SCI population.20 Recent body composition studies in the elderly, for which disabled populations are often used as a model, suggest that body fatness and particularly abdominal obesity are independent risk factors for cardiovascular disease14 and insulin resistance.13 In addition, it appears that abdominal fat, and not muscle, contributes to glucose intolerance.12 The role of IMAT for metabolic disease risk is not completely characterized.26 Current literature on body composition in the SCI population is not adequate enough to establish which tissue (reductions in lean vs increases in fat) contributes most to risk for metabolic disease. Nevertheless, although our findings suggest that abdominal obesity and thigh IMAT accumulation relate to an increased risk for metabolic disease in the SCI population, high level physical training protects against glucose intolerance and insulin resistance compared to AB controls.
Surprisingly, we found little group differences and very few associations between body composition measures and the lipid profile in our sample. The lack of association between blood lipids and body composition and lack of differences in blood lipids between SCI and AB may be partly explained by the relatively low adiposity and similar body fat percentage in both groups. In addition, our subjects were young healthy individuals.
HDL-C levels in this study were of particular interest, because physical activity is known to increase HDL-C levels in the general population,27 and low HDL-C levels in SCI individuals are often attributed to physical inactivity.28, 29 Although there was no significant difference between the groups in our study, the mean HDL-C level in the SCI athletes was 53 compared to 46 mg/dl in the sedentary AB controls, and this is well above HDL-C levels ranging between 32 and 45 mg/dl reported previously in sedentary SCI individuals.28, 29 In the AB population exercise training has shown to increase HDL-C levels by 2–8 mg/dl,27 which is approximately the difference in mean HDL-C we found and levels reported previously in the SCI population. Possibly, with a larger sample size we may have been able to show a significant difference between groups. Indeed, the effect size for HDL-C was moderate at ∼0.60.
The main limitation in this study is the lack of a sedentary SCI comparison group; however, there are several published studies with this population to draw comparisons with, as we have aimed to do in this discussion. The SCI individuals in this study were trained athletes, which does not reflect the typical physical activity level of most individuals with SCI. Another limitation is the lack of a measure of visceral adiposity using MRI, which was prohibited due to major artifacts in MRI abdominal scans. The hypothetical error due to metal implants in the DXA trunk fat measurements is unknown but assumed negligible. Lastly, our sample size is relatively small impacting our statistical power.
In summary, although glucose tolerance and lipid profile did not differ significantly between SCI athletes and AB controls, data from this study suggest that upper-body physical activity can improve insulin sensitivity and HDL-C levels in the SCI population compared to sedentary AB individuals. Furthermore, whole body, abdominal adiposity and thigh IMAT all appear to impact risk for metabolic disease in SCI individuals.
References
Midha M, Schmitt JK, Sclater M . Exercise effect with the wheelchair aerobic fitness trainer on conditioning and metabolic function in disabled persons: a pilot study. Arch Phys Med Rehabil 1999; 80: 258–261.
Duran FS, Lugo L, Ramirez L, Eusse E . Effects of an exercise program on the rehabilitation of patients with spinal cord injury. Arch Phys Med Rehabil 2001; 82: 1349–1354.
Bizzarini E, Saccavini M, Lipanje F, Magrin P, Malisan C, Zampa A . Exercise prescription in subjects with spinal cord injuries. Arch Phys Med Rehabil 2005; 86: 1170–1175.
Manns PJ, McCubbin JA, Williams DP . Fitness, inflammation, and the metabolic syndrome in men with paraplegia. Arch Phys Med Rehabil 2005; 86: 1176–1181.
Chen Z, Lohman TG, Stini WA, Ritenbaugh C, Aickin M . Fat or lean tissue mass: which one is the major determinant of bone mineral mass in healthy postmenopausal women? J Bone Miner Res 1997; 12: 144–151.
Inukai Y, Takahashi K, Wang DH, Kira S . Assessment of total and segmental body composition in spinal cord-injured athletes in Okayama prefecture of Japan. Acta Medica Okayama 2006; 60: 99–106.
de Groot PC, Hjeltnes N, Heijboer AC, Stal W, Birkeland K . Effect of training intensity on physical capacity, lipid profile and insulin sensitivity in early rehabilitation of spinal cord injured individuals. Spinal Cord 2003; 41: 673–679.
El-Sayed MS, Younesian A . Lipid profiles are influenced by arm cranking exercise and training in individuals with spinal cord injury. Spinal Cord 2005; 43: 299–305.
Shen W, Wang Z, Punyanita M, Lei J, Sinav A, Kral JG et al. Adipose tissue quantification by imaging methods: a proposed classification. Obes Res 2003; 11: 5–16.
Gallagher D, Kuznia P, Heshka S, Albu J, Heymsfield SB, Goodpaster B et al. Adipose tissue in muscle: a novel depot similar in size to visceral adipose tissue. Am J Clin Nutr 2005; 81: 903–910.
Song MY, Ruts E, Kim J, Janumala I, Heymsfield S, Gallagher D . Sarcopenia and increased adipose tissue infiltration of muscle in elderly African American women. Am J Clin Nutr 2004; 79: 874–880.
Kohrt WM, Holloszy JO . Loss of skeletal muscle mass with aging: effect on glucose tolerance. J Gerontol 1995; 50: 68–72.
Racette SB, Evans EM, Weiss EP, Hagberg JM, Holloszy JO . Abdominal adiposity is a stronger predictor of insulin resistance than fitness among 50–95 year olds. Diab Care 2006; 29: 673–678.
Van Pelt RE, Jankowski CM, Gozansky WS, Schwartz RS, Kohrt WM . Lower-body adiposity and metabolic protection in postmenopausal women. J Clin Endocrinol Metab 2005; 90: 4573–4578.
Goodpaster BH, Krishnaswami S, Resnick H, Kelley DE, Haggerty C, Harris TB et al. Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diab Care 2003; 26: 372–379.
Goodpaster BH, Krishnaswami S, Harris TB, Katsiaras A, Kritchevsky SB, Simonsick EM et al. Obesity, regional body fat distribution, and the metabolic syndrome in older men and women. Arch Intern Med 2005; 165: 777–783.
Modlesky CM, Bickel CS, Slade JM, Meyer RA, Cureton KJ, Dudley GA . Assessment of skeletal muscle mass in men with spinal cord injury using dual-energy X-ray absorptiometry and magnetic resonance imaging. J Appl Physiol 2004; 96: 561–565.
Elder CP, Apple DF, Bickel CS, Meyer RA, Dudley GA . Intramuscular fat and glucose tolerance after spinal cord injury--a cross-sectional study. Spinal Cord 2004; 42: 711–716.
Friedewald WT, Levy RI, Fredrickson DS . Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972; 18: 499–502.
Bauman WA, Kahn NN, Grimm DR, Spungen AM . Risk factors for atherogenesis and cardiovascular autonomic function in persons with spinal cord injury. Spinal Cord 1999; 37: 601–616.
Bauman WA, Spungen AM . Carbohydrate and lipid metabolism in chronic spinal cord injury. J Spinal Cord Med 2001; 24: 266–277.
Karlsson AK . Insulin resistance and sympathetic function in high spinal cord injury. Spinal Cord 1999; 37: 494–500.
Mohr T, Dela F, Handberg A, Biering-Sorensen F, Galbo H, Kjaer M . Insulin action and long-term electrically induced training in individuals with spinal cord injuries. Med Sci Sport Exerc 2001; 33: 1247–1252.
Jeon JY, Weiss CB, Steadward RD, Ryan E, Burnham RS, Bell G et al. Improved glucose tolerance and insulin sensitivity after electrical stimulation-assisted cycling in people with spinal cord injury. Spinal Cord 2002; 40: 110–117.
Snijder MB, Visser M, Dekker JM, Goodpaster BH, Harris TB, Kritchevsky SB et al. Low subcutaneous thigh fat is a risk factor for unfavourable glucose and lipid levels, independently of high abdominal fat. The Health ABC Study. Diabetologia 2005; 48: 301–308.
Dube J, Goodpaster BH . Assessment of intramuscular triglycerides: contribution to metabolic abnormalities. Curr Opin Clin Nutr Metab Care 2006; 9: 553–559.
Durstine JL, Grandjean PW, Cox CA, Thompson PD . Lipids, lipoproteins, and exercise. J Cardiopulm Rehabil 2002; 22: 385–398.
Bauman WA, Adkins RH, Spungen AM, Herbert R, Schechter C, Smith D et al. Is immobilization associated with an abnormal lipoprotein profile? Observations from a diverse cohort. Spinal Cord 1999; 37: 485–493.
Washburn RA, Figoni SF . High density lipoprotein cholesterol in individuals with spinal cord injury: the potential role of physical activity. Spinal Cord 1999; 37: 685–695.
Acknowledgements
We thank the study participants for co-operation. We also thank Ron Meyer for allowing us to use Winvessel for analyzing the MR images, and Jill Slade for her help in using the software. This study was supported in part by Project Export Grant awarded to University of Illinois at Rockford by the NIH, National Center on Minority Health and Health Disparities (NCMHD), University of Illinois MJ Neer Grant, and the Schneider Grant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mojtahedi, M., Valentine, R., Arngrímsson, S. et al. The association between regional body composition and metabolic outcomes in athletes with spinal cord injury. Spinal Cord 46, 192–197 (2008). https://doi.org/10.1038/sj.sc.3102076
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.sc.3102076
Keywords
This article is cited by
-
Endocrinological and inflammatory markers in individuals with spinal cord injury: A systematic review and meta-analysis
Reviews in Endocrine and Metabolic Disorders (2022)
-
Skeletal muscle hypertrophy and attenuation of cardio-metabolic risk factors (SHARC) using functional electrical stimulation-lower extremity cycling in persons with spinal cord injury: study protocol for a randomized clinical trial
Trials (2019)
-
Nutrition education for cardiovascular disease prevention in individuals with spinal cord injuries: study protocol for a randomized controlled trial
Trials (2017)