Abstract
The olfactory bulbectomized (OB) rat is a well-characterized animal model that exhibits a number of behavioral and neurochemical changes that have relevance to clinical depression. Hyperactivity in the open field is the most widely used parameter assessed in this model and is reversed following chronic, but not acute, antidepressant treatment. This study investigated OB-induced alterations in heart rate, body temperature, and neuronal activation following open-field exposure and the impact of chronic treatment with fluoxetine on these parameters. Upon placement in the open field, OB rats exhibited a characteristic hyperactivity response. Heart rate and body temperature were increased in sham-operated rats following open-field exposure, a predictable response to stress, which was significantly reduced in OB rats. Moreover bulbectomy reduced open field-induced cFOS expression in the basal nucleus of the stria terminalis while concurrently increasing expression in the hippocampus, amygdala, paraventricular nucleus of the thalamus, and dorsal raphe nucleus. Chronic fluoxetine treatment (10 mg/kg subcutaneous once daily for 5 weeks) attenuated all of these OB-associated changes. In conclusion, OB rats exhibit alterations in behavior, body temperature, heart rate, and neuronal activation in response to open-field exposure, which are reversed following chronic fluoxetine administration. These results identify stress-sensitive regions within the brain which are altered following bulbectomy and which may underlie the abnormal behavioral and physiological changes observed in this rodent model of depression.
Similar content being viewed by others
INTRODUCTION
As depression is causally linked to stressful life events (Kendler et al, 2001), exposure to stressful events is often employed in attempts to model depression and anxiety disorders in animals. A well-documented and validated model of depression is the olfactory bulbectomized (OB) rat, which exhibits a number of behavioral, neurochemical, neuroendocrine, and immune alterations correlating with changes observed in depressed patients (Kelly et al, 1997; Harkin et al, 2003; Song and Leonard, 2005). Hyperactivity on exposure to a stressful open-field environment is the most commonly assessed behavioral change in the model, a response which is attenuated following chronic, but not acute, antidepressant treatments (Kelly et al, 1997).
Exposure to a novel environment is regarded as a mild psychological stressor eliciting an increase in temperature and heart rate, increased plasma corticosterone levels, and behavioral activation in naive animals (Van Den Buuse et al, 2001, 2002; Harkin et al, 2002a). Although studies have demonstrated neuroendocrine (Marcilhac et al, 1999) and neurochemical (Connor et al, 1999; Ho et al, 2000; Masini et al, 2004) activation concurrently with behavioral alterations in the OB rat, a paucity of data exists regarding assessment of physiological parameters in the model. This is of particular interest as a number of physiological changes such as reduced heart rate variability, altered autonomic tone and sleep disturbances are known to occur during stress and depression (Van Den Buuse et al, 2001, 2002; Carney et al, 2001; Grippo and Johnson, 2002).
The behavioral changes associated with the OB model have been proposed to be owing to neuronal reorganization and plasticity following removal of the olfactory bulbs (Grecksch et al, 1997). Although many critical brain regions have demonstrated adaptational changes in the model (Nesterova et al, 1997; Wrynn et al, 2000a), the neuroanatomical regions involved in OB-induced hyperactivity have not been defined. The immediate early gene, c-fos is induced in response to stressful stimuli and has been extensively used as a tool to map neuronal functional activation (Kovacs, 1998). For example, exposure of rats to a stressful environment increases c-fos expression in cortical areas (medial prefrontal, cingulate, orbital, parietal), olfactory bulb, lateral septum (LS), amygdala, hippocampus, thalamus, caudate, hypothalamus, and periaquaductal grey (Handa et al, 1993; Mulders et al, 1995; Nagahara and Handa, 1997; Wirtshafter et al, 1998; Babai et al, 2001; Klejbor et al, 2003). Furthermore, antidepressants have been shown to modify stress-induced expression of c-fos (Morinobu et al, 1995; Duncan et al, 1996).
The aim of this study was to examine changes in activity, temperature, and heart rate following open-field exposure in OB rats. A further objective was to identify if specific brain regions are differentially activated in response to open-field exposure following OB when compared to sham-operated controls. This experimental approach was employed to determine the regions associated with the characteristic OB-related response to a stressful challenge. The effect of repeated treatment with the prototypical antidepressant fluoxetine on behavioral and physiological parameters and neuronal activation in the OB rat was also assessed.
MATERIALS AND METHODS
Subjects
Experiments were conducted on male Sprague–Dawley rats (weight at start of experiment 220–270 g; Harlan, UK), housed singly in a plastic-bottomed cage (45 × 25 × 20 cm) containing wood shavings as bedding. The animals were maintained at a constant temperature (20±2°C) and at standard lighting conditions (12 : 12 h light–dark, lights on from 0800 to 2000 h). Food and water were available ad libitum. The experimental protocol was carried out in accordance with the guidelines of the Animal Welfare Committee, National University of Ireland, Galway under licence from the Irish Department of Health and Children and in compliance with the European Communities Council directive 86/609.
Bilateral Olfactory Bulbectomy Surgery
Bilateral OB was performed on rats anaesthetized with 7.5% chloral hydrate (375 mg/kg i.p.: Merck, Germany) using an injection volume of 5 ml/kg, essentially as previously outlined by Van Riezen and Leonard (1990). In brief, the head was shaven and a midline sagittal incision was made in the skin overlying the skull. Two burr holes of 2 mm diameter were drilled into the skull, 5 mm rostral to bregma, and 2 mm lateral to the midline. The olfactory bulbs were removed by gentle aspiration with a water vacuum pump and care was taken not to damage the frontal cortex (FC). The burr holes were then plugged with a hemostatic sponge to control bleeding. Sham-operated animals were treated in the same manner but the bulbs were left intact. All animals were allowed 7 days to recover following surgery before undergoing implantation of bioradiotelemetric transponders (see below). Lesions were verified after completion of the study. Animals were eliminated from the analysis if the bulbs were not completely removed or if damage extended to the FC. Sham-operated animals were removed if there was any damage to the bulbs or the FC.
Implantation of Bioradiotelemetric Transponders
Body temperature (°C), heart rate (beats per minute (BPM)), and locomotor activity (counts per minute (CPM)) were recorded by bioradiotelemetry using fully implantable series PDT-4000 E-mitter transponders and Vital View (Mini Mitter Co., OR, USA) as previously described (Harkin et al, 2002a, 2002b). One week following OB, rats were anesthetized with 7.5% chloral hydrate (375 mg/kg i.p) using an injection volume of 5 ml/kg. A small abdominal incision was made and the transponders were implanted into the abdominal cavity along the sagittal plane, dorsal to the digestive organs. Animals were allowed 1 week to recover before drug treatment. Previous studies demonstrate that locomotor activity does not differ between transponder-implanted, sham-operated, or naive rats 3 weeks post-surgery (Harkin et al, 2002b).
Drug Treatment
Rats received the selective serotonin (5HT) re-uptake inhibitor (SSRI), fluoxetine (Clonmel Chemicals, Ireland) (10 mg/kg/day), or vehicle (distilled H2O). Fluoxetine was administered to rats by the subcutaneous route owing to the presence of the radiotelemetric transponder in the abdominal cavity. Drug treatment continued for a period of 5 weeks. Performance in the open field was assessed 24 h following the last drug administration in order to minimize any acute effect of drug administration.
Open-Field Test
Each animal was removed from the home cage and placed singly into a brightly lit (lux 200–250) novel open-field environment (diameter 75 cm) where locomotor activity was assessed using an electronic video tracking system (Noldus EthoVision, Version 3.0) for a 15-min period. Locomotor activity (distance moved: cm) was monitored in minute intervals for the entire duration of the test period. Following exposure to the open field, rats were returned to their home cages and telemetric data were collected for a further 75 min.
cFOS Immunohistochemistry
Rats were deeply anesthetized with chloral hydrate (800 mg/kg i.p.) 75 min after the end of the open-field stress and transcardially perfused with 100 ml of heparinized (5 IU/ml) saline solution, followed by 500 ml of 4% (w/v) paraformaldehyde in 0.1 M phosphate buffer (PB) at pH 7.4 and 4°C. Brains were removed and stored in the same fixative for 90 min at 4°C followed by immersion in 20% (w/v) sucrose solution in 0.1 M PB containing 1% (w/v) sodium azide (NaAz) for at least 24 h. Brains were rapidly frozen on dry ice and coronal sections of 40 μm were cut on a cryostat and collected in 0.1 M PB.
Before immunohistochemical staining sections were washed in 0.1 M PB and then placed in 0.75% (v/v) hydrogen peroxide (H2O2) for 20 min in order to quench the endogenous peroxides in the tissue. cFOS immunolabelling was performed using a polyclonal antibody directed against residues 4–17 of human c-fos (Calbiochem, MerckBiosciences, Nottingham, UK). In brief, sections were incubated for 24 h at room temperature under constant agitation in 0.1 M PB-saline containing cFOS antisera raised in rabbit (1 : 20 000), 0.3% (v/v) Triton X, 0.04% (w/v) bovine serum albumin, and 0.1% (w/v) NaAz. The incubated sections were washed and incubated for 90 min in biotinylated donkey-anti-rabbit antisera (1 : 200; Jackson ImmunoResearch Europe, UK). The secondary antibody had minimal cross-reactivity to non-target species. This was followed by incubation in the avidin–biotin–peroxidase complex (1 : 600; ABC Elite Kit; Vector Laboratories Ltd, Peterborough, UK) for a further 90 min followed by sections being immersed in 0.02% (w/v) 3,3-diaminobenzidine-4HCl (DAB) containing 0.01% (v/v) H2O2 in PB for 10–15 min for a brown reaction product that was terminated by rinses in PB. The sections were mounted on glass-gelatinized slides and air dried. All sections were lightly counterstained with cresyl violet, dehydrated in graded alcohols, cleared with xylene, and coversliped with DePex mounting medium. Photomicrographs were taken with an Olympus microscope BX51 and Olympus C5060 digital camera (Mason Technology, Dublin, Ireland).
Quantification of cFOS Profiles
cFOS-positive nuclei were quantified from a fixed area size using a Kodak ID (Version 3.5) image analysis software. The system was calibrated to ignore background staining. The group identity of the rat was obscured during the counting procedure. Regions of interest were defined based on the extent of cellular groups comprising specific landmarks in accordance with Paxinos and Watson (1986) rat brain atlas. The planes of the sections were standardized as far as possible. The anterior–posterior (AP) level from bregma of the regions was analyzed as follows: FC (AP: 0.20 mm), lateral septum (LS) (AP: 0.20 mm), basal nucleus of the stria terminalis (BNST: AP: −0.26 mm), paraventricular nucleus of the hypothalamus (PVN; AP: −2.12 mm), central nucleus of the amygdala (CeAMY; AP: −2.8 mm), basolateral amygdala (BLA; AP: −2.8 mm), paraventricular nucleus of the thalamus (PVT; AP: −2.8 mm), Dentate Gyrus (DG; AP: −2.8 mm), CA1 region of the hippocampus (CA1; AP: −2.8 mm), habenula (AP: −3.14 mm), dorsal raphe nucleus (DRN; AP: −8.0 mm), locus coeruleus (AP: −9.68 mm). cFOS immunoreactive profiles were counted from a fixed area under 100–200 × in at least two sections per region, bilaterally and averaged to give the mean number of cFOS-positive profiles for that region per animal.
Statistical Analysis
Data were analyzed by analysis of variance (ANOVA) using a GB-STAT (Version 8) statistical package. Distance travelled in the open field was analyzed in 1-min time bins over the entire course of the 15-min trial by repeated measures ANOVA with lesion (Sham and OB), drug treatment (vehicle and fluoxetine), and time as factors. Additional analyses were performed on data totalled over the first 5 min of the trial using two-way ANOVA with lesion and drug treatment as factors. Body temperature and heart rate were continuously assessed, except during the open-field test, on account of a technical limitation of the telemetric receiving devices, and an average computed at 5-min intervals for 30 min before and 75 min following open-field exposure. For determination of the change in temperature and heart rate, a baseline was calculated by averaging samples collected before open-field exposure, which was subtracted from all values to determine change from baseline average. Locomotor activity data were assessed as the sum of the activity counts per 5 min interval. Data post-stressor exposures were compared to the baseline average. Analyses were performed using repeated measures ANOVA with lesion, drug treatment, and time as factors. Neuronal activation data were analyzed by two-way ANOVA with lesion and drug treatment as factors. All inter-group comparisons were assessed using a Student Newman Keuls post hoc comparison test where appropriate. Data are expressed as mean±SEM and were deemed significant when P<0.05.
RESULTS
Chronic Fluoxetine Treatment Attenuates OB-Induced Hyperactivity
ANOVA of locomotor activity over the 15-min open-field test showed an effect of lesion (F1,16=6.17, P=0.024), lesion × drug treatment interaction (F1,16=5.83, P=0.028), time (F14,224=15.08, P<0.0001), lesion × time interaction (F14,224=5.37, P<0.0001) and drug treatment × time interaction (F14,224=2.00, P=0.019). Post hoc comparisons revealed that vehicle-treated OB rats display an increase in distance travelled in the first and third minute of open-field exposure when compared to sham-operated controls (Figure 1a). OB-induced hyperactivity was attenuated by chronic fluoxetine treatment. Fluoxetine treatment did not alter locomotor activity of sham-operated animals when compared to vehicle-treated controls. Analysis of behavior over the first 5 min of the test trial demonstrated a lesion (F1,19=14.77, P=0.001) and lesion × drug interaction effect (F1,19=7.06, P=0.017). OB induced an increase in distance travelled in the open field when compared to sham-operated controls (Figure 1b). Chronic fluoxetine treatment did not affect behavioral responses of sham-operated rats but attenuated OB-induced hyperactivity in the test arena when compared to their vehicle-treated OB counterparts (Figure 1b).
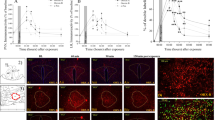
The effect of bulbectomy and chronic fluoxetine treatment on distance travelled (cm) over (a) the entire 15-min open-field exposure and (b) the first 5 min in the open-field arena. Data expressed as means±SEM (n=5–6). **P<0.01 compared to vehicle-treated sham-operated controls. +P<0.05, ++P<0.01 compared to vehicle-treated OB group.
Open Field-Induced Hyperthermia is Attenuated in OB Rats; Normalization by Chronic Fluoxetine Treatment
No lesion, drug treatment, or interaction effects were found on baseline temperature before open-field exposure (Figure 2a). ANOVA of core body temperature following the open-field test showed an effect of time (F15,270=44.65, P<0.001), lesion × time interaction (F15,270=7.36, P<0.001), and drug treatment × time interaction (F15,270= 4.08, P<0.001). Post hoc analysis revealed that body temperature of sham-operated controls was increased from the 20th to 60th minute interval when compared to their pre-stress baseline levels (Figure 2a). This robust hyperthermic response to open-field exposure in sham-operated animals was not altered by chronic fluoxetine treatment. In contrast, open-field exposure did not increase core body temperature of OB rats from baseline level. However, fluoxetine-treated OB rats exhibited a hyperthermic response to open-field exposure for the 20th–70th minute interval. In addition, OB rats demonstrated a reduced hyperthermic response following open-field exposure for the 20th–45th minute when compared to sham-operated controls. Chronic fluoxetine treatment normalized the temperature response of OB rats when compared to their vehicle-treated counterparts (Figure 2a).
The effect of OB and chronic fluoxetine treatment on open field-induced changes in (a) core body temperature, (b) heart rate and (c) locomotor activity. N=5–6 per group. Data expressed as means±SEM of mean change from baseline for temperature and heart rate recorded at 5-min intervals. Data expressed as mean±SEM of sum of counts per 5-min interval for locomotor activity. *P<0.05, **P<0.01 compared to vehicle-treated sham-operated controls. +P<0.05, ++P<0.01 compared to vehicle-treated OB group. Baseline averages for body temperature (Sham+Vehicle: 36.78±0.23°C; Sham+Fluoxetine: 37.11±0.30°C; OB+Vehicle: 36.55±0.10°C; OB+Fluoxetine: 36.50±0.21°C), heart rate (Sham+Vehicle: 334±7 BPM; Sham+Fluoxetine: 319±25 BPM; OB+Vehicle: 337±19 BPM; OB+Fluoxetine: 334±16 BPM) and locomotor activity (Sham+Vehicle: 6±1 CPM; Sham+Fluoxetine: 3±2 CPM; OB+Vehicle: 5±2 CPM; OB+Fluoxetine: 6±2 CPM). □ Sham+Vehicle ○ Sham+Fluoxetine ▪ OB+Vehicle • OB+Fluoxetine.
Open Field-Induced Tachycardia is Attenuated in OB Rats; Reversal Following Chronic Fluoxetine Treatment
No lesion, drug treatment, or interaction effects were found on baseline heart rate before open-field exposure (Figure 2b). ANOVA of heart rate following the open-field test showed an effect of lesion (F1,19=15.33, P<0.001), time (F15,270=20.39, P<0.001), lesion × time interaction (F15,270=1.73, P=0.044), and drug treatment × time interaction (F15,270=1.87, P<0.026). Post hoc analysis revealed that sham-operated animals exhibited an increase in heart rate in response to open-field exposure over the 20th–90th minute interval when compared to their pre-stress baseline (Figure 2b). This tachycardic response was not altered by chronic fluoxetine treatment. Heart rate of OB rats was increased for the 20th–40th minute interval following the open-field test when compared to baseline levels. In comparison, a robust tachycardic response was obtained in OB rats following fluoxetine treatment over the 20th–70th minute interval following the open-field test when compared to pre-stress baseline. OB rats demonstrated an attenuated tachycardic response following open-field exposure for the 25th–30th, 40th, and 65th–70th minute when compared to sham-operated controls. Chronic fluoxetine treatment blocked the reduced tachardiac response of OB rats in the 65th minute when compared to their vehicle-treated counterparts (Figure 2b).
Home Cage Locomotor Activity
No lesion, drug treatment, or interaction effects were found on baseline home cage activity open-field exposure (Figure 2c). On returning to the home cage following the open-field test, home cage activity was increased in both sham and OB animals for the first 5 min when compared to baseline scores (F15,270=33.57, P<0.001). A slower acclimatization was observed in the fluoxetine-treated groups where increased activity was observed over the 20th–35th min interval when compared to baseline scores (F15,270=2.04, P=0.013) (Figure 2c).
Differential cFOS Expression in the OB Rat Following Open-Field Exposure
ANOVA showed an effect of lesion on cFOS expression following the open-field test in several discrete brain regions when compared to sham-operated controls. An effect of bulbectomy lesion was observed in the BNST (F1,19=6.20, P=0.024), central (CeAMY; F1,20=4.69, P=0.046), and basolateral nucleus (BLA; F1,20=8.60, P=0.009) of the amygdala, PVT (F1,19=6.20, P=0.024), dentate gyrus (DG; F1,22=7.11, P=0.015), and CA1 region (F1,21=6.77, P=0.018) of the hippocampus, and DRN (F1,20=9.18, P=0.008). Post hoc comparisons revealed that cFOS expression was increased in the CeAMY, BLA, PVT, DG, CA1, and DRN of OB rats when compared to sham-operated controls (Figures 3 and 4). Concurrently a decrease in cFOS activation was observed in the BNST in the OB rat when compared to sham-operated controls. Fluoxetine treatment did not alter cFOS expression in any brain region when compared to vehicle-treated sham-operated rats. However, altered cFOS expression in the OB brain following open-field exposure was attenuated by chronic fluoxetine treatment (BNST (F1,21=8.07, P=0.011), CeAMY (F1,20=6.25, P=0.023), BLA (F1,20=8.60, P=0.009), DG (F1,22=5.27, P=0.033), PVT (F1,19=7.20, P=0.016); CA1 (F1,21=4.70, P=0.044), DRN (F1,20=4.60, P=0.047)) when compared to their vehicle-treated counterparts (Figure 3). cFOS expression in the FC (Sham Vehicle (367±56), Sham Fluoxetine (437±44), OB Vehicle (395±41), OB Fluoxetine (459±78)), LS (Sham Vehicle (263±16), Sham Fluoxetine (244±26), OB Vehicle (249±18), OB Fluoxetine (221±41)), PVN (Sham Vehicle (82±6), Sham Fluoxetine (93±12), OB Vehicle (62±11), OB Fluoxetine (88±13)), habenula ((Sham Vehicle (21±2), Sham Fluoxetine (22±3), OB Vehicle (24±2), OB Fluoxetine (20±3)) or locus coeruleus ((Sham Vehicle (49±5), Sham Fluoxetine (35±5), OB Vehicle (45±5), OB Fluoxetine (35±10)) was not effected by bulbectomy lesion or drug treatment.
The mean number (+SEM) of cFos immunoreactive profiles in the brain of sham or OB rats chronically treated with vehicle or fluoxetine. N=5–6 per group. BNST—Basal nucleus of the stria terminalis; CeAMY—central nucleus of the amygdala; BLA—basolateral nucleus of the amygdala; PVT—paraventricular nucleus of the thalamus; DG—dentate gyrus; CA1—CA1 regions of the hippocampus; DRN—dorsal raphe nucleus. *P<0.05, **P<0.01 compared to vehicle-treated sham-operated controls. +P<0.05, ++P<0.01 compared to vehicle-treated OB group.
Photomicrographs of cFOS expression in three brain regions in vehicle-treated sham-operated control and OB rats. BNST (a) sham (b) OB; CeAMY (c) sham (d) OB and LW (e) sham (f) OB. Abbreviations: 3V—3rd ventricle, BNST—basolateral nucleus of the stria terminalis, CeAMY—central nucleus of the amygdala, DRN—dorsal raphe nucleus, LW—lateral wing of the DRN, LV—lateral ventricle, VR—ventral regions of DRN. Bar represents 0.1 mm.
DISCUSSION
This study demonstrates that OB-induced hyperactivity in the open field is accompanied by impaired temperature and heart rate responses. This provides behavioral and physiological evidence that OB rats are unable to mount appropriate responses to a stressful stimulus. Additionally, this is the first study to show that neuronal activation is increased in the amygdala (CeAMY and BLA), hippocampus (CA1 and DG), thalamus (PVT), and DRN of the OB rat upon exposure to a stressful environment. This is accompanied by a decrease in open field-induced cFOS expression in the BNST. Chronic fluoxetine treatment attenuated alterations in behavior, temperature and heart rate and central cFOS expression in the OB rat following open-field exposure.
Stressor exposure elicits a predictable physiological stress response characterized by increased temperature, heart rate, and blood pressure (Van Den Buuse et al, 2001, 2002; Harkin et al, 2002a). The present findings provide evidence that OB rats are unable to increase core body temperature following open-field exposure. Novelty-induced hyperthermia is a fever response owing to an elevated thermoregulatory set point (Oka et al, 2001). Although the mechanisms underlying the temperature response of OB rats to stressor exposure remain unresolved, OB rats may have a lower internal thermoregulatory set point, resulting in these animals being unable to mount an appropriate hyperthermic response to open-field exposure. In addition, a reduced heart rate response to the open field was also observed in these animals. Although physiological responses were not assessed during the open-field test previous studies have demonstrated reduced heart rate activation in the first 15 min of novelty exposure 5 and 10 days following bulbectomy (Kawasaki et al, 1980). Cardiovascular responses to novelty are due to sympathetic activation and/or vagal withdrawal (Van Den Buuse et al, 2001, 2002), alterations in which may account for the reduced heart rate response to the open field in the OB rat. A reduced cardiosympathetic response was reported on exposure of OB rats to other stressors, namely hypotension, air jet stress, and smoke exposure (Moffitt et al, 2002). Thus, removal of the olfactory bulbs may result in autonomic dysfunction correlating with alterations also observed in the clinical setting (Carney et al, 2001; Grippo and Johnson, 2002). The reduced temperature and heart rate responses were concurrently observed with increased activity in the test arena suggesting that the physiological responses to open-field exposure are independent of activity levels. Thus, separate anatomical pathways may modulate behavioral and physiological responses to stress in the model.
Chronic fluoxetine treatment attenuated the OB-related hyperactivity in the open field and the impaired physiological responses to open-field exposure. However, owing to the long half-lives of fluoxetine and its active metabolite norfluoxetine (Caccia et al, 1990; Gardier et al, 1994; Lefebvre et al, 1999) the acute effects of fluoxetine administration on cannot be completely ruled out. It should be noted that although the impaired temperature response of OB rats to the open-field test was completely normalized by fluoxetine treatment, the effect of this treatment regimen on the decreased heart rate response of OB rats was not as pronounced. A longer treatment period with this SSRI may be necessary to fully attenuate the lesion-induced reduction in heart rate response.
Mapping the pattern of neuronal activation in response to open-field exposure identifies critical brain regions in the OB rat possibly involved in the hyperactivity and reduced temperature and heart rate responses observed in the model. Removal of the olfactory bulbs is associated with ventricular enlargement, decreased cortical, hippocampal, caudate and amygdaloid volumes, and disruption of the blood–brain barrier (Wrynn et al, 2000a), results which correlate with clinical studies in the depressed patient (Videbech, 1997; Sheline, 2000; Drevets, 2000). The olfactory bulbs send direct connections to the cortex (pyriform, entorhinal, and parahippocampal), amygdala and BNST (Haberly and Price, 1977; Leonard and Tuite, 1981). Many of the behavioral alterations observed in the OB rat have been attributed to severed connections between the olfactory organs and various brain centers, with much emphasis being placed on the resultant disinhibition of the amygdala following removal of the bulbs (Leonard and Tuite, 1981; Van Riezen and Leonard, 1990; McNish and Davis, 1997; Mucignat-Caretta et al, 2004). Increased neuronal activation in the CeAMY and BLA of the OB rat following open-field exposure confirms that the amygdaloid nuclei are integral in mounting a stress response in these animals. Altered gene expression (Wrynn et al, 2000b) and cell proliferation (Keilhoff et al, 2006) has been previously reported in the BLA of OB rats and this region has been proposed as a potential site of antidepressant activity. The decrease in cFOS expression in the BNST of OB rat would suggest that this area is not activated in response to open-field exposure in the model. However, it is possible that cells of this region are activated by processes that do not promote the synthesis of the cFOS protein.
The BNST has been implicated in mediating responses relating more to anxiety and apprehension than fear, whereas the CeAMY is involved in the initiation and maintenance of fear, and perhaps to a lesser extent anxiety (Davis and Shi, 1999; Lang et al, 2000; Walker et al, 2003). Differential activation of neuronal networks in these limbic regions may indicate that exposure of OB rats to a novel environment induced an emotional stress response mediated, in part, by fear via neuronal activation of the CeAMY. In addition, reduced neuronal activation in the BNST may indicate that OB rats exhibit a reduced state of defensive preparedness and anxiety to stressor exposure. This correlates with previous reports of reduced defensive behavior in OB rats (Primeaux and Holmes, 1999; Stock et al, 2001).
The amygdala and BNST receive inputs from the hippocampus and PVT (Cullinan et al, 1993, Bubser and Deutch, 1999), both of which also exhibit increased cFOS expression in the OB rat brain following open-field exposure. In turn, the amygdaloid complex projects to the PVN and brain stem nuclei such as the central gray and dorsal motor nucleus of the vagus nerve (Gray and Magnuson, 1987; Gray et al, 1989). Owing to these extensive anatomical connections, neural processing involving the BNST and CeAMY controls much of the behavioral, physiological, neuroendocrine, and autonomic responses to stress (Davis and Shi, 1999; Van de Kar and Blair, 1999; Carrasco and Van de Kar, 2003). Thus, the pattern of open field-induced changes in neuronal activation following OB suggest that the neural circuit mediating stress responses are altered in this model, accounting for the behavioral and physiological responses observed. Chronic fluoxetine treatment acts on neuronal pathways in these discrete brain regions to attenuate the maladaptive response of OB rats to stress as measured by changes in cFOS expression, behavioral activity, and reduced temperature and heart rate responses.
Further studies are required in order to determine the neurotransmitter or transmitter systems associated with these alterations. In this regard, the OB rat has been proposed as a model of hyposerotonergic depression with many of the behavioral and biochemical aspects of the model also observed following destruction of serotonergic innervation of the bulbs (Lumia et al, 1992). Bulbectomy induces a reterograde loss of cells in the DRN (Nesterova et al, 1997), decreased basal 5HT levels and rate of 5HT synthesis in the amygdala and hippocampus (Marcilhac et al, 1999; van der Stelt et al, 2005), increased 5HT2A receptor density and sensitivity in the FC (Gurevich et al, 1993; Nakagawasai et al, 2003) and increased density of 5HT transporter sites in the cortex and hippocampus (Slotkin et al, 1999). In response to stress, OB rats demonstrate an increase in 5HT neurotransmission in the hypothalamus (Marcilhac et al, 1999) and nucleus accumbens (Connor et al, 1999). Combining these earlier studies with our evidence for increased neuronal activation in the DRN and other forebrain regions highly innervated by the raphe nuclei, the central serotonergic system may be implicated in mediating the stress responses to open-field exposure observed in the model. Moreover, the attenuation of altered neuronal activation in the OB rat by chronic treatment with the SSRI fluoxetine lends further support for the involvement of the DRN-5HT system in stress-induced changes in activity, temperature, and heart rate in the OB rat model of depression. However, uncertainty remains as to whether the effects observed with fluoxetine extend to antidepressants with different acute modes of action.
In conclusion, this study identifies stress-sensitive neuroanatomical regions, which are altered in the OB rat. Altered neuronal expression in these regions reflects changes in the central stress circuitry in the model and may underlie the behavioral and physiological changes observed on exposure to the open field. Moreover these studies indicate neuroanatomical regions that may underlie antidepressant activity in the model.
References
Babai P, Anokhin KV, Dolgov N, Sudakov KV (2001). Characteristics of c-fos gene expression in the brains of rats with different investigative and defensive behaviors. Neurosci Behav Physiol 31: 583–588.
Bubser M, Deutch AY (1999). Stress induces Fos expression in neurons of the thalamic paraventricular nucleus that innervate limbic forebrain sites. Synapse 32: 13–22.
Caccia S, Cappi M, Fracasso C, Garattini S (1990). Influence of dose and route of administration on the kinetics of fluoxetine and its metabolite norfluoxetine in the rat. Psychopharmacology (Berlin) 100: 509–514.
Carney RM, Blumenthal JA, Stein PK, Watkins L, Catellier D, Berkman LF et al (2001). Depression, heart rate variability, and acute myocardial infarction. Circulation 104: 2024–2028.
Carrasco GA, Van de Kar LD (2003). Neuroendocrine pharmacology of stress. Eur J Pharmacol 463: 235–272.
Connor TJ, Song C, Leonard BE, Anisman H, Merali Z (1999). Stressor-induced alterations in serotonergic activity in an animal model of depression. Neuroreport 10: 523–528.
Cullinan WE, Herman JP, Watson SJ (1993). Ventral subicular interaction with the hypothalamic paraventricular nucleus: evidence for a relay in the bed nucleus of the stria terminalis. J Comp Neurol 332: 1–20.
Davis M, Shi C (1999). The extended amygdala: are the central nucleus of the amygdala and the bed nucleus of the stria terminalis differentially involved in fear versus anxiety? Ann NY Acad Sci 877: 281–291.
Drevets WC (2000). Neuroimaging studies of mood disorders. Biol Psychiatry 48: 813–829.
Duncan GE, Knapp DJ, Johnson KB, Breese GR (1996). Functional classification of antidepressants based on antagonism of swim stress-induced fos-like immunoreactivity. J Pharmacol Exp Ther 277: 1076–1089.
Gardier AM, Lepoul E, Trouvin JH, Chanut E, Dessalles MC, Jacquot C (1994). Changes in dopamine metabolism in rat forebrain regions after cessation of long-term fluoxetine treatment: relationship with brain concentrations of fluoxetine and norfluoxetine. Life Sci 54: PL51–PL56.
Gray TS, Carney ME, Magnuson DJ (1989). Direct projections from the central amygdaloid nucleus to the hypothalamic paraventricular nucleus: possible role in stress-induced adrenocorticotropin release. Neuroendocrinology 50: 433–446.
Gray TS, Magnuson DJ (1987). Neuropeptide neuronal efferents from the bed nucleus of the stria terminalis and central amygdaloid nucleus to the dorsal vagal complex in the rat. J Comp Neurol 262: 365–374.
Grecksch G, Zhou D, Franke C, Schroder U, Sabel B, Becker A et al (1997). Influence of olfactory bulbectomy and subsequent imipramine treatment on 5-hydroxytryptaminergic presynapses in the rat frontal cortex: behavioural correlates. Br J Pharmacol 122: 1725–1731.
Grippo AJ, Johnson AK (2002). Biological mechanisms in the relationship between depression and heart disease. Neurosci Biobehav Rev 26: 941–962.
Gurevich EV, Aleksandrova IA, Otmakhova NA, Katkov YA, Nesterova IV, Bobkova NV (1993). Effects of bulbectomy and subsequent antidepressant treatment on brain 5-HT2 and 5-HT1A receptors in mice. Pharmacol Biochem Behav 45: 65–70.
Haberly LB, Price JL (1977). The axonal projection patterns of the mitral and tufted cells of the olfactory bulb in the rat. Brain Res 129: 152–157.
Handa RJ, Nunley KM, Bollnow MR (1993). Induction of c-fos mRNA in the brain and anterior pituitary gland by a novel environment. Neuroreport 4: 1079–1082.
Harkin A, Connor TJ, O'Donnell JM, Kelly JP (2002a). Physiological and behavioural responses to stress: What does a rat find stressful? Lab Animal Europe 2: 32–40.
Harkin A, Kelly JP, Leonard BE (2003). A review of the relevance and validity of olfactory bulbectomy as a model of depression. Clin Neurosci Res 3: 253–262.
Harkin A, O'Donnell JM, Kelly JP (2002b). A study of vital view for behavioural and physiological monitoring in laboratory rats. Physiol Behav 77: 65–77.
Ho YJ, Chang YC, Liu TM, Tai MY, Wong CS, Tsai YF (2000). Striatal glutamate release during novelty exposure-induced hyperactivity in olfactory bulbectomized rats. Neurosci Lett 287: 117–120.
Kawasaki H, Watanabe S, Ueka S (1980). Changes in blood pressure and heart rate following bilateral olfactory bulbectomy in rats. Physiol Behav 24: 51–56.
Keilhoff G, Becker A, Grecksch G, Bernstein HG, Wolf G (2006). Cell proliferation is influenced by bulbectomy and normalized by imipramine treatment in a region-specific manner. Neuropsychopharmacology 31: 1165–1176.
Kelly JP, Wrynn AS, Leonard BE (1997). The olfactory bulbectomized rat as a model of depression: an update. Pharmacol Ther 74: 299–316.
Kendler KS, Thornton LM, Gardner CO (2001). Genetic risk, number of previous depressive episodes, and stressful life events in predicting onset of major depression. Am J Psychiatry 158: 582–586.
Klejbor I, Luczynska A, Ludkiewicz B, Domaradzka-Pytel B, Morys J (2003). The developmental pattern of c-fos expression in the rat thalamus following open-field stress stimulation. Pol J Vet Sci 6: 201–207.
Kovacs KJ (1998). c-Fos as a transcription factor: a stressful (re)view from a functional map. Neurochem Int 33: 287–297.
Lang PJ, Davis M, Ohman A (2000). Fear and anxiety: animal models and human cognitive psychophysiology. J Affect Disord 61: 137–159.
Lefebvre M, Marchand M, Horowitz JM, Torres G (1999). Detection of fluoxetine in brain, blood, liver and hair of rats using gas chromatography-mass spectrometry. Life Sci 64: 805–811.
Leonard BE, Tuite M (1981). Anatomical, physiological, and behavioral aspects of olfactory bulbectomy in the rat. Int Rev Neurobiol 22: 251–286.
Lumia AR, Teicher MH, Salchli F, Ayers E, Possidente B (1992). Olfactory bulbectomy as a model for agitated hyposerotonergic depression. Brain Res 587: 181–185.
Marcilhac A, Faudon M, Anglade G, Hery F, Siaud P (1999). An investigation of serotonergic involvement in the regulation of ACTH and corticosterone in the olfactory bulbectomized rat. Pharmacol Biochem Behav 63: 599–605.
Masini CV, Holmes PV, Freeman KG, Maki AC, Edwards GL (2004). Dopamine overflow is increased in olfactory bulbectomized rats: an in vivo microdialysis study. Physiol Behav 81: 111–119.
McNish KA, Davis M (1997). Olfactory bulbectomy enhances sensitization of the acoustic startle reflex produced by acute or repeated stress. Behav Neurosci 111: 80–91.
Moffitt JA, Grippo AJ, Holmes PV, Johnson AK (2002). Olfactory bulbectomy attenuates cardiovascular sympathoexcitatory reflexes in rats. Am J Physiol Heart Circ Physiol 283: H2575–H2583.
Morinobu S, Nibuya M, Duman RS (1995). Chronic antidepressant treatment down-regulates the induction of c-fos mRNA in response to acute stress in rat frontal cortex. Neuropsychopharmacology 12: 221–228.
Mucignat-Caretta C, Bondi M, Caretta A (2004). Animal models of depression: olfactory lesions affect amygdala, subventricular zone, and aggression. Neurobiol Dis 16: 386–395.
Mulders WH, Meek J, Schmidt ED, Hafmans TG, Cools AR (1995). The hypothalamic paraventricular nucleus in two types of Wistar rats with different stress responses. II. Differential Fos-expression. Brain Res 689: 61–70.
Nagahara AH, Handa RJ (1997). Age-related changes in c-fos mRNA induction after open-field exposure in the rat brain. Neurobiol Aging 18: 45–55.
Nakagawasai O, Tadano T, Arai Y, Hozumi S, Oba A, Tan-No K et al (2003). Enhancement of 5-hydroxytryptamine-induced head-twitch response after olfactory bulbectomy. Neuroscience 117: 1017–1023.
Nesterova IV, Gurevich EV, Nesterov VI, Otmakhova NA, Bobkova NV (1997). Bulbectomy-induced loss of raphe neurons is counteracted by antidepressant treatment. Prog Neuropsychopharmacol Biol Psychiatry 21: 127–140.
Oka T, Oka K, Hori T (2001). Mechanisms and mediators of psychological stress-induced rise in core temperature. Psychosom Med 63: 476–486.
Paxinos G, Watson C (1986). The Rat Brain in Stereotaxic Co-ordinates, 2nd edn. Academic Press Limited: New York.
Primeaux SD, Holmes PV (1999). Role of aversively motivated behavior in the olfactory bulbectomy syndrome. Physiol Behav 67: 41–47.
Sheline YI (2000). 3D MRI studies of neuroanatomic changes in unipolar major depression: the role of stress and medical comorbidity. Biol Psychiatry 48: 791–800.
Slotkin TA, Miller DB, Fumagalli F, McCook EC, Zhang J, Bissette G et al (1999). Modeling geriatric depression in animals: biochemical and behavioral effects of olfactory bulbectomy in young versus aged rats. J Pharmacol Exp Ther 289: 334–345.
Song C, Leonard BE (2005). The olfactory bulbectomised rat as a model of depression. Neurosci Biobehav Rev 29: 627–647.
Stock HS, Hand GA, Ford K, Wilson MA (2001). Changes in defensive behaviors following olfactory bulbectomy in male and female rats. Brain Res 903: 242–246.
Van de Kar LD, Blair ML (1999). Forebrain pathways mediating stress-induced hormone secretion. Front Neuroendocrinol 20: 1–48.
van den Buuse M, Van Acker SA, Fluttert M, De Kloet ER (2001). Blood pressure, heart rate, and behavioral responses to psychological ‘novelty’ stress in freely moving rats. Psychophysiology 38: 490–499.
van den Buuse M, van Acker SA, Fluttert MF, de Kloet ER (2002). Involvement of corticosterone in cardiovascular responses to an open-field novelty stressor in freely moving rats. Physiol Behav 75: 207–215.
van der Stelt HM, Breuer ME, Olivier B, Westenberg HG (2005). Permanent deficits in serotonergic functioning of olfactory bulbectomized rats: an in vivo microdialysis study. Biol Psychiatry 57: 1061–1067.
Van Riezen H, Leonard BE (1990). Effects of psychotropic drugs on the behavior and neurochemistry of olfactory bulbectomized rats. Pharmacol Ther 47: 21–34.
Videbech P (1997). MRI findings in patients with affective disorder: a meta-analysis. Acta Psychiatr Scand 96: 157–168.
Walker DL, Toufexis DJ, Davis M (2003). Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol 463: 199–216.
Wirtshafter D, Stratford TR, Shim I (1998). Placement in a novel environment induces fos-like immunoreactivity in supramammillary cells projecting to the hippocampus and midbrain. Brain Res 789: 331–334.
Wrynn AS, Mac Sweeney CP, Franconi F, Lemaire L, Pouliquen D, Herlidou S et al (2000a). An in-vivo magnetic resonance imaging study of the olfactory bulbectomized rat model of depression. Brain Res 879: 193–199.
Wrynn AS, Sebens JB, Koch T, Leonard BE, Korf J (2000b). Prolonged c-Jun expression in the basolateral amygdala following bulbectomy: possible implications for antidepressant activity and time of onset. Brain Res Mol Brain Res 76: 7–17.
Acknowledgements
This work was presented at the European College of Neuropsychopharmacology (ECNP) Workshop, Nice, France, March 2005 and European College of Neuropsychopharmacology (ECNP) Congress, Amsterdam, Netherlands, October 2005.
We thank Dr David Finn for providing helpful comments on the preparation of this manuscript. Technical assistance from Daniel Kerr is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roche, M., Harkin, A. & Kelly, J. Chronic Fluoxetine Treatment Attenuates Stressor-Induced Changes in Temperature, Heart Rate, and Neuronal Activation in the Olfactory Bulbectomized Rat. Neuropsychopharmacol 32, 1312–1320 (2007). https://doi.org/10.1038/sj.npp.1301253
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.npp.1301253
Keywords
This article is cited by
-
An altered spinal serotonergic system contributes to increased thermal nociception in an animal model of depression
Experimental Brain Research (2014)
-
Leading compounds for the validation of animal models of psychopathology
Cell and Tissue Research (2013)
-
Analysis of morphological changes as a key method in studying psychiatric animal models
Cell and Tissue Research (2013)
-
Neuronal NOS Inhibitor and Conventional Antidepressant Drugs Attenuate Stress-induced Fos Expression in Overlapping Brain Regions
Cellular and Molecular Neurobiology (2012)
-
Chronic therapy with citalopram decreases regional cerebral glucose utilization in OBX, and not sham-operated, rats: an autoradiographic study
Psychopharmacology (2009)