Abstract
Nitric oxide (NO) plays a major role in cardiopulmonary regulation as illustrated by the alterations of the NO system described in cardiopulmonary illnesses. Recent studies have found an association between panic disorder and cardiovascular death and illness, as well as pulmonary diseases. Our objective was to investigate whether pulmonary or systemic NO production was altered during induced panic attacks (PAs). We used a double-blind placebo-controlled crossover design with randomization of the order of an injection of placebo and pentagastrin, a cholecystokinin-B receptor agonist that induces PAs in healthy volunteers (HVs). A total of 17 HVs experienced a PA after pentagastrin challenge. Exhaled NO and NO metabolites were measured by chemiluminescence. During pentagastrin-induced PAs, HVs displayed significant decreases in plateau concentrations of NO exhaled, which were associated with proportional increases in minute ventilation. There were no significant changes in pulmonary or systemic NO production. These results suggest that the decrease in exhaled NO concentration observed during pentagastrin-induced PAs is related to the associated hyperventilation, rather than to any change in lung NO production. This study is the first to evaluate changes in NO measurements during acute anxiety.
Similar content being viewed by others
INTRODUCTION
The vascular endothelium synthesizes and releases several relaxing factors, including nitric oxide (NO). NO is synthesized from the amino acid L-arginine by a family of enzymes known as nitric oxide synthases (NOS). Three isoforms of NOS have been identified and cloned: the neuronal (nNOS) and endothelial (eNOS) constitutive isoforms and the inducible (iNOS) isoform. NO is widely accepted as an important biomediator involved in the regulation of vascular and bronchial tone (Moncada et al, 1991; Zapol et al, 1994). NO has an important role in the pathophysiologic processes leading to cardiovascular and pulmonary diseases, such as reduction in NO endothelial release in atherosclerotic vessels (Cooke and Tsao, 1994) and increased NO production in respiratory diseases such as asthma (Hamid et al, 1997).
Panic disorder (PD) is characterized by recurrent and unexpected periods of intense fear accompanied by symptoms of a panic attack (PA) such as tremor, palpitations, dyspnea, dizziness, paresthesias, gastric sensations, hot flushes and/or cold chills, diaphoresis, and chest pain or discomfort (American Psychiatric Association, 1994).
In the last two decades, PD has been associated with increased cardiovascular mortality and morbidity (Coryell et al, 1982; Coryell, 1988; Weissman et al, 1990; Kawachi et al, 1994). Furthermore, there is a high association between pulmonary illnesses and PD, as evidenced by the increased prevalence of asthma in PD patients (Spinhoven et al, 1994). Respiratory alterations such as persistent respiratory irregularity and increased respiratory response to the respiratory stimulant doxapram have also been reported in PD patients (Abelson et al, 2001). Dysregulation of the NO system in PD patients may ultimately contribute to the increased cardiovascular risk and the respiratory dysfunction associated with PD. The exact pathophysiologic mechanism of respiratory alterations in panic remains unclear. For instance, it has been hypothesized that the dyspnea experienced by PD patients may be the result of the triggering of a ‘false suffocation alarm’, suggesting a central abnormality in the control of ventilation (Klein, 1993). According to this theory, dyspnea would be the key symptom in the chain of events that lead to a PA. Endogenously produced NO plays an integral role in the physiological regulation of many airway functions such as bronchodilation and regulation of airway and pulmonary blood flow (Barnes and Belvisi, 1993); we therefore hypothesized that abnormal NO production could play a role in the occurrence of respiratory symptoms featured during PAs.
Cholecystokinin (CCK) type B receptor agonists such as tetragastrin (CCK-4) and pentagastrin (CCK-5) have been widely used to investigate the neurobiological changes taking place during PAs. Intravenous bolus injections of CCK-4 and CCK-5 induce short-lived PAs in PD patients and to a lesser degree in healthy volunteers (HVs) (panic response rate 70–100% and 17–70%, respectively) (Van Megen et al, 1996). These PAs are reported by PD patients to be very similar to the naturally occurring PA.
The direct measurement of plasma NO concentration remains difficult in vivo because NO is rapidly inactivated by hemoglobin or oxidized to form several nitrogen dioxides (NOx). Plasma NOx concentration is used as a marker for endogenous NO production in humans (Moncada et al, 1991). Another valid method of measurement of endogenous NO production is to assess the NO excreted in the exhaled air, detected by a chemiluminescence assay (Gustafsson et al, 1991; Kharitonov et al, 1997; Leone et al, 1994; Dillon et al, 1996; Archer et al, 1998).
We tested the hypothesis that CCK-5-induced PAs in HVs will be associated with increased respiratory rate, tidal volume, and alteration of NO production in exhaled breath and plasma.
METHODS
Subjects
A total of 24 normotensive subjects, taking no medications and with no evidence of medical conditions at the time of the study, were administered the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) Axis I Disorders (SCID-I) and were deemed eligible based on the lack of Axis I psychiatric disorders. As in previous studies, we used the Panic Symptom Scale (PSS) to assess the intensity of the panic response to CCK-5 and to differentiate nonpanickers from panickers (those meeting criteria for occurrence of a PA) (Bradwejn et al, 1991). In all, 17 out of 24 nonsmoker subjects who received CCK-5 were defined as panickers (71%) and included in the study (13 males and four females mean age±SEM 24.71±1.65 years). Female subjects were tested during their early follicular phase. The research protocol was approved by the Health Research Ethics Board and all subjects provided written consent.
Study Design
A double-blind placebo-controlled crossover study was undertaken. Subjects were randomized to receive intravenous 5 s-bolus of either placebo first and 3–7 days later CCK-5 (50 μg) (CLINALFA, Läufelfingen, Switzerland), or CCK-5 first and placebo 3–7 days later.
General Procedures
Subjects followed a nitrate/nitrite restricted diet (Wang et al, 1997) for 3 days before the test and fasted overnight (12 h) (Mochizuki et al, 2000). At 45 min before injection, an intravenous catheter was inserted into the antecubital vein for drug infusion and blood collection. Shortly after, a face mask occluding the nose was placed on the subject. Measurements were taken breath by breath from baseline (30 s interval taken before injection) up to 3 min 40 s postinjection. Calculations of tidal volume, respiratory rate, and minute/ventilation were performed using Power-lab software (AD Instruments; Castle Hill, Australia).
NO in Exhaled Breath Measurement
[NO]exh was measured as previously described (Archer, 1993), using chemiluminescence analysis (NO analyzer 280; Sievers Instruments, Boulder, CO). Ambient NO contamination was avoided by the use of NO-free air (<3 ppb of NO). To exclude nasal air, known to contain high levels of NO from nasal and paranasal origin (Archer et al, 1998), a nonrebreathing face mask was used (Hans Rudolph Inc.; Kansas City, MO). Lung NO production (VNO) by both endothelial and epithelial cells was calculated by

where VE is the minute ventilation, 0.826 is the gases universal constant (Riley et al, 1997), and [NO]exh is the exhaled NO concentration. To obtain NO released from the lower airways (pulmonary endothelium), we calculated the plateau seen in [NO]exh, as previously described in other studies (Kharitonov et al, 1996,1997; Rutgers et al, 1998).
Classically, the mean function (m) has been used to obtain the plateau (defined as the interval from 1/2 to 7/8 of the duration of the exhalation) of the exhaled concentration of NO

where t1⩽ti⩽tN and [t0, tN] is the interval of time of the plateau, N is the number of observations, and X(ti) is the value of the exhaled NO concentration at time ti.
However, variations in the respiratory pattern will not be detected by this calculation and some important information can be missed. For this reason, we used the integral function (i) on the plateau on each breath

where f(t) is the exhaled NO concentration (ppb) at time t and [t0, tf] is the interval of time of the plateau (s). Therefore, more information about the behavior of the curve can be obtained, especially when the measurements of NO are carried out breath by breath with variable expiratory flows (Figure 1).
Results obtained with both methods, [NO]exh (m) and [NO]exh (i) will be presented.
NOx Measurement
NOx were measured by chemiluminescence in small volumes of plasma (10 μl) by converting them to NO using a strong reducing environment: vanadium (iii) in hydrochloric acid 1 N at 90°C (Archer, 1993).
STATISTICAL ANALYSIS
Following the experimental design, a linear model for crossover design (Jones and Kenward, 1989) was used to analyze the data. The following parameters were included in the model: the main effect of time, the main effect of treatment, the main effect of visit (first vs second injection visit), the main effect of order of treatment (CCK-5 first vs CCK-5s), and the following interactions: treatment with time, visit with time, and order of treatment with time. A p-value of less than 5% was considered significant. Bonferroni's multiple comparisons analyses were also carried out, where appropriate. All statistical analyses were conducted using SAS statistical software for Windows, Release 8.02 (SAS Institute Inc., Cary, NC)
RESULTS
The PSS score after CCK-5 injection was greater than after placebo injection (PSS Score means±SEM of 29.65±2.72 after CCK-5 injection and 1.24±0.58 after placebo injection. p<0.001).
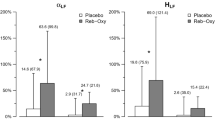
A significant decrease in plateau [NO]exh levels, for both calculations of integral function [NO]exh (i) (Figure 2) and mean [NO]exh (m) was found after pentagastrin-induced PA, but not with placebo, as illustrated by time effect and treatment × time effect (Table 1). Bonferroni's post hoc analysis showed a statistically significant decrease (p<0.05) in [NO]exh (m) at 40 s, 1 min, and 1 min 20 s compared to baseline after CCK-5-induced PAs, and at 40 and 1 min in [NO]exh (i). Statistically significant treatment by time interactions were found in tidal volume (Figure 3), respiratory rate (Figure 4), and minute ventilation (Figure 2) (defined as the product of tidal volume and respiratory frequency) after CCK-5-induced PAs (Table 1). Bonferroni's post hoc analysis showed significant increases (p<0.05) compared to baseline, in minute ventilation (Figure 2) and respiratory frequency (Figure 4) at 40 s, 1 min, and 1 min 20 s after CCK-5-induced PAs, and at 40 s in tidal volume (Figure 3). However, there were no statistically significant time, treatment, and time × treatment effects in plateau NO production (VNO) (integral function and mean) after pentagastrin-induced PAs (Table 1). No significant changes were noted in plasma NOx after CCK-5-induced PAs (Table 1).
No order of visit effect or order of treatment effect was observed on any of the variables.
DISCUSSION
In the present study, administration of CCK-5 induced intense panic symptoms compared to placebo. This is in accordance with previous CCK-5 panic challenge investigations performed in HVs (Van Megen et al, 1996). Previous studies have shown that PD patients display an increased panic response to CCK-4 and CCK-5 (Bradwejn et al, 1995; Van Megen et al, 1994), suggesting that CCK plays a role in the neurobiology of PD, either through an increase in central CCK neural activity or an increase in CCK receptor sensitivity (Lydiard et al, 1992). However, the exact mechanism leading to the occurrence of PAs remains unclear.
In the present investigation, significant increases in tidal volume (VT), respiratory frequency (RR), and minute ventilation (VE) were found during CCK-5-induced PAs. These findings differ from a previous research report using CCK-4 in HVs. Bradwejn et al (1998) showed that CCK-4-induced panic symptoms produced an increase in VE mediated by increases in VT, but not in RR. This discrepancy regarding the increase in RR between our study and Bradwejn et al's could be explained by the fact that we studied only panickers (17), whereas Bradwejn et al (1998) studied nonpanickers (six) and panickers (nine) together. In their discussion, Bradwejn et al noted that the VE in panickers appear to be greater than in nonpanickers, but the observed difference did not reach statistical significance; however, they did not provide information on RR in panickers vs nonpanickers. Our findings of an increase in RR during CCK-5-induced PA are also supported by results of other studies that used different panic challenges and that showed an increase in VT and RR during induced PAs (Gorman et al, 1988,2001). Interestingly, Goetz et al (1993) have described a marginally significant increase in RR during PA induced by placebo infusion in PD patients, with a peak of the RR around the same time (30 s) as the peak in RR that we observed (40 s) (Figure 4). Another human study testing the effects of CCK-4 on airway resistance found no differences in spirometric variables after CCK-4-induced panic symptoms (Shlik et al, 1997). Findings from these studies and our results suggest that the CCK-induced changes in respiratory function have a central rather than peripheral mechanism.
The present study is the first to investigate the changes in measurements of NO during induced anxiety/panic in humans. We found that CCK-5-induced PA produced a significant decrease in exhaled NO levels, without affecting NO production (VNO). These findings can be explained by an increase in minute ventilation such that, with constant VNO, there is a dilutional decrease in [NO]exh. Interestingly, these ventilatory changes and associated decreased [NO]exh were concomitant and occurred at the time of maximum intensity of panic symptoms.
Dilutional decreases in [NO]exh have also been found in other situations such as exercise. During exercise, there is a decrease in [NO]exh and an increase in VNO for both tidal breathing and end-tidal plateau measurements of NO (Phillips et al, 1996). The decrease in exhaled NO concentration may be due to large increases in airflow rates, reducing the contact time between the airway wall and the exhaled air (St. Croix et al, 1999). Despite the decreased [NO]exh concentration, the large increases in ventilation during exercise can result in increased VNO. The decrease in [NO]exh concentration observed during exercise can also be explained by the rapid uptake of NO by the blood, due to increased diffusion of NO. However, no changes in NO metabolites (NOx) in plasma have been found during heavy exercise (St. Croix et al, 1999). In contrast, in our study, no significant changes in NO production (VNO and NOx) were found during CCK-5-induced PAs. The phenomena observed during PA may be similar to those of exercise, except that there was no increase in NO production but a stable NO production resulting from balanced changes in VE and [NO]exh.
Increased rates of cardiovascular/cerebrovascular morbidity (Weissman et al, 1990) and mortality (mortality ratio for men suffering from PD was twice that of the predicted ratio) (Coryell et al, 1986) have been described in PD patients. This increase in cardiovascular/cerebrovascular morbidity and mortality rates may be due to the existence of chronic cardiovascular (CV) risk in PD patients, illustrated either by elevated conventional risk factors such as smoking (Amering et al, 1999) or by chronic physiological states such as a sympathetic predominance as suggested by the decreased heart rate variability consistently described in PD patients (Yeragani et al, 1998). It appears that the increased risk for sudden cardiac death found in patients with high levels of phobic anxiety, a common feature in PD patients, persists even after correction for conventional risk factors (Kawachi et al, 1994). The CV risk and particularly the increased risk for sudden cardiac death associated with PD, is therefore also likely due to the acute physiological and neurochemical disturbances occurring during a PA. For example, specific panic symptoms such as palpitations, sweating, and shaking suggest an activation of the sympathetic system that could promote the occurrence of cardiac arrhythmia. We have, indeed, shown that pretreatment with intravenous propranolol blunts the panic response to CCK-4-induced PA (Le Mellédo et al, 1998). The respiratory alterations that accompany a PA (such as hyperventilation) observed in our study could also contribute to the acute CV risk during PA. Indeed, hyperventilation has been described to potentially precipitate coronary spasm in the presence or absence of atherosclerosis (Mansour et al, 1998). From our peripheral measurements of NO in HV; however, it appears that lung NO does not play a major role in respiratory changes associated with acute PAs.
In summary, this is the first study to establish that the increased minute ventilation observed during CCK-B-agonists-induced PAs was due to increases in both tidal volume and respiratory rate. We have also shown that during CCK-5-induced PAs, there is a decrease in NO concentration in exhaled breath with no alteration of pulmonary or systemic NO production. This decrease in [NO]exh appears to be due to an increase in minute ventilation. However, these results need to be replicated in patients with PD who are known to display a greater panic response to CCK-B receptor agonists than HVs. Although it has been suggested that NO has an important role in the central modulation of respiration, the present results only assessed the possible connection between peripheral actions of NO (such as cardiopulmonary regulation) and respiratory symptoms displayed during PAs; therefore, we cannot make any inference regarding the role of brain NO in the pathophysiology of PAs.
References
Abelson JL, Weg JG, Nesse RM, Curtis GC (2001). Persistent respiratory irregularity in patients with panic disorder. Biol Psychiatry 49: 588–595.
American Psychiatric Association (1994). DSM-IV: Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Press: Washington, DC.
Amering M, Bankier B, Berger P, Griengl H, Windhaber J, Katschnig H (1999). Panic disorder and cigarette smoking behavior. Compr Psychiatry 140: 35–38.
Archer SL (1993). Measurement of nitric oxide in biological models. FASEB J 7: 349–360.
Archer SL, Djaballah K, Humbert M, Weir KE, Fartoukh M, Dall'ava-Santucci J et al (1998). Nitric Oxide deficiency in fenfluramine- and dexfenfluramine-induced pulmonary hypertension. Am J Respir Crit Care Med 158: 1061–1067.
Barnes PJ, Belvisi MG (1993). Nitric oxide and lung disease. Thorax 48: 1034–1043.
Bradwejn J, LeGrand J-M, Koszycki D, Bates JHT, Bourin M (1998). Effects of cholecystokinin tetrapeptide on respiratory function in healthy volunteers. Am J psychiatry 155: 280–282.
Bradwejn J, Koszycki D, Paradis M, Reece P, Hinton J, Sedman A (1995). Effect of CI-988 on cholecystokinin tetrapeptide-induced panic symptoms in healthy volunteers. Biol Psychiatry 38: 742–746.
Bradwejn J, Koszycki D, Shriqui C (1991). Enhanced sensitivity to cholecystokinin tetrapeptide in panic disorder; clinical and behavioral findings. Arch Gen Psychiatry 48: 603–610.
Cooke JP, Tsao PS (1994). Is NO an endogenous antiatherogenic molecule? Arteriosclet Thromb 14: 653–655.
Coryell W (1988). Panic disorder and mortality. Psychiatr Clin North Am 11: 433–440.
Coryell W, Noyes R, Clancy J (1982). Excess mortality in panic disorder. Arch Gen Psychiatry 39: 701–703.
Coryell W, Noyes R, Hause JD (1986). Mortality among outpatients with anxiety disorders. Am J Psychiatry 143: 508–510.
Dillon WC, Hampl V, Schultz PJ, Rubins JB, Archer SL (1996). Origins of breath nitric oxide in humans. Chest 110: 930–938.
Goetz RR, Klein DF, Kahn J, Liebowitz MR, Fyer AJ, Gorman JM (1993). Panic attacks during placebo procedures in the laboratory. Physiology and symptomatology. Arch Gen Psychiatry 50: 280–285.
Gorman JM, Goetz R, Martinez J, Uy J, Ross D, Fyer AJ et al (1988). Hyperventilation occurs during lactate-induced panic. J Anxiety Disord 2: 193–202.
Gorman JM, Kent J, Martinez J, Browne S, Coplan J, Papp LA (2001). Physiological changes during carbon dioxide inhalation in patients with panic disorder, major depression and premenstrual dysphoric syndrome. Arch Gen Psychiatry 58: 125–131.
Gustafsson LE, Leone AM, Person MG, Wiklund NP, Moncada S (1991). Endogenous nitric oxide is present in the exhaled air of rabbits, guinea pigs and humans. Biochem Biophys Res Commun 181: 852–857.
Hamid Q, Springall DR, Riveros-Moreno V (1997). Induction of nitric oxide synthase in asthma. J Allergy Clin Immunol 150: 624–629.
Jones B, Kenward MG (1989). Design and Analysis of Cross-Over Trials. Chapman & Hall: New York.
Kawachi I, Colditz GA, Ascherio A, Rimm EB, Giovanucci E, Stampfer MJ et al (1994). Prospective study of phobic anxiety and risk of coronary heart disease in men. Circulation 89: 1992–1997.
Kharitonov S, Alving K, Barnes PJ (1997). Exhaled and nasal nitric oxide measurements: recommendations. Eur Res J 10: 1683–1693.
Kharitonov S, Chung KF, Evans DJ, O'Connor BJ, Barnes PJ (1996). Increased exhaled nitric oxide in asthma is derived from the lower respiratory tract. Am J Respir Crit Care Med 153: 1773–1780.
Klein DF (1993). False suffocation alarms, spontaneous panic and related conditions: an integrative hypothesis. Arch Gen Psychiatry 50: 306–317.
Le Mellédo J-M, Bradwejn J, Koszycki D, Bichet DG, Bellavance F (1998). The role of the B-noradrenergic system in cholecystokinin-tetrapeptide-induced panic symptoms. Biol Psychiatry 44: 364–366.
Leone AM, Gustafsson LE, Francis PL, Persson MG, Wiklund NP, Moncada S (1994). Nitric oxide in exhaled breath in humans: direct GC-MS confirmation. Biochem Biophys Res Commun 201: 883–887.
Lydiard RB, Ballenger JC, Laraie MT, Fossey MD, Beinfeld MC (1992). CSF cholecystokinin concentrations in patients with panic disorder and normal comparison subjects. Am J Psychiatry 149: 691–693.
Mansour VM, Wilkinson DJC, Jennings GL, Schwarz RG, Thompson JM, Esler MD (1998). Panic disorder: coronary spasm as a basis for cardiac risk? Med J Aust 168: 390–392.
Mochizuki S, Toyota E, Hiramatsu O, Kajita T, Shigeto F, Takemoto M et al (2000). Effect of dietary control on plasma nitrate level and estimation of basal systemic nitric oxide production rate in humans. Heart Vessels 15: 274–279.
Moncada S, Palmer RMJ, Higgs EA (1991). Nitric oxide: physiology, pathophysiology and pharmacology. Pharmacol Rev 43: 109–143.
Phillips CR, Giraud GD, Holden WE (1996). Exhaled nitric oxide during exercise: site of release and modulation by ventilation and blood flow. J Appl Physiol 80: 1865–1871.
Riley MS, Pórszász J, Miranda J, Engelen MPKJ, Brundage B, Wasserman K (1997). Exhaled nitric oxide during exercise in primary pulmonary hypertension and pulmonary fibrosis. Chest 111: 44–50.
Rutgers SR, Meijer RJ, Kerstjens HAM, van der Mark ThW, Koëter GH, Postma DS (1998). Nitric oxide measured with single-breath and tidal-breathing methods in asthma and COPD. Eur Respir J 12: 816–819.
Shlik J, Vasar V, Aluoja A, Kingisepp P-H, Jagomägi K, Vasar E et al (1997). The effect of cholecystokinin tetrapeptide on respiratory resistance in healthy volunteers. Biol Psychiatry 42: 206–212.
Spinhoven P, Ros M, Westgeest A, Van der Does AJ (1994). The prevalence of respiratory disorders in panic disorder, major depressive disorder and V-code patients. Behav Res Ther 32: 647–649.
St. Croix CM, Wetter TJ, Pegelow DF, Meyer KC, Dempsey JA (1999). Assessment of nitric oxide formation during exercise. Am J Respir Crit Care Med 159: 1125–1133.
Van Megen HJGM, Westenberg HGM, den Boer JA, Haigh JRM, Traub M (1994). Pentagastrin induced panic attacks: enhanced sensitivity in panic disorder patients. Psychopharmacology 114: 449–455.
Van Megen HJGM, Westenberg HGM, den Boer JA, Kahn RS (1996). Cholecystokinin in anxiety. Eur Neuropsychopharmacol 6: 263–280.
Wang J, Brown MA, Tam SH, Chan MC, Whitworth JA (1997). Effects of diet on measurement of NO metabolites. Clin Exp Pharmacol Physiol 24: 418–420.
Weissman MM, Markowitz JS, Oulette R, Greenwald S, Kahan JP (1990). Panic disorder and cardiovascular/cerebrovascular problems: results from a community survey. Am J Psychiatry 147: 1504–1508.
Yeragani VK, Sobolewski E, Igel G, Johnson C, Jampala VC, Kay J et al (1998). Decreased heart-period variability in patients with panic disorder: a study of Holter ECG records. Psychiatry Res 78: 89–99.
Zapol WM, Rimar S, Gillis N (1994). Nitric oxide and the lung. Summary of an NHLBI workshop. Am J Respir Crit Care Med 149: 1375–1380.
Acknowledgements
We thank Janisse Khudabux RN, Tom Ryan, Ross Waite, and Kyoko Hashimoto for their collaboration in the development and execution of the study. Also, we would like to thank Gustavo Carrero MSc for mathematical assistance and advice. This research was sponsored by an operating grant of the University of Alberta Hospital Foundation to JMLM and SA as well as by a Canadian Institute of Health Research (CIHR) grant to JMLM. JMLM is an AHFMR (Alberta Heritage Foundation For Medical Research) clinical investigator. SA is an AHFMR scientist, supported by the Heart and Stroke Foundation of Alberta.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lara, N., Chrapko, W., Archer, S. et al. Pulmonary and Systemic Nitric Oxide Measurements During CCK-5-Induced Panic Attacks. Neuropsychopharmacol 28, 1840–1845 (2003). https://doi.org/10.1038/sj.npp.1300241
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.npp.1300241