Abstract
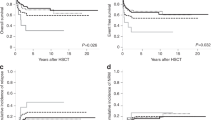
Genotype and immunophenotype can be used to define biological species of acute lymphoblastic leukemia (ALL). The purpose of these two pilot studies, conducted between 1986 and 1994, was to explore the feasibility and acceptability of classifying ALL in this manner for selection of treatment rather than using conventional risk for failure factors such as age and initial white blood cell count. The possibility that conventional risk factors would be overcome and survival improved by this approach was also considered. Flow cytometry and chromosome analysis were used to classify the ALL of 150 children into one of five biologic categories as defined by cell surface antigens, DNA index and chromosome number and arrangement. Chemotherapy regimens depended on the assigned category. There was no provision for cranial irradiation and use of alkylating agents, anthracyclines and epipodophyllotoxins was restricted in order to reduce risk of late adverse sequelae. All patients are included in the analysis regardless of presenting condition or adherence to protocol. The majority of patients were Mexican–American or African–American. Eight-year event-free survival (EFS) is 60.7% (±4%) and 8-year overall survival (OAS) 72.6% (±3.7%). EFS and OAS varied significantly among the biologic categories despite differences in chemotherapy regimens. When the patients with B-precursor ALL were retrospectively classified by current Pediatric Oncology Group (POG) criteria, 8-year EFS was 82% (±7.3%) for the good risk group, 68.9% (±5.9%) for the standard risk and 48.8% (±7.6%) for the poor risk, all significant differences. However, when retrospectively classified according to the Rome/NCI prognostic criteria the 8-year EFS for standard risk patients was 69.1% (±5.1%) and for high risk 58.8% (±6.9%), not a statistically significant difference. Numbers of T cell and B cell patients are too few for comparison. Gender and ethnicity influenced survival as in treatment based on prognostic factors. Initial central nervous system (CNS) relapse occurred in five patients (3%) and combined CNS and hematological relapse in six (3%). Factors significantly associated with CNS and combined relapse were leukemic pleocytosis in the initial CSF sample, pro-B immunophenotype and DNA index <1.16, but not initial white blood cell count. Only three survivors appear to have serious late adverse sequelae, the only neurologic the result of asparaginase-induced cortical vein thrombosis. The results suggest that use of biologic species as defined by immunophenotype and genotype to select therapy of all is feasible and acceptable but under the conditions of these studies offered no apparent therapeutic advantage over conventional risk grouping. However, the introduction of molecular genotyping and novel gene targeted therapeutic agents justify further exploration of this approach.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Aur RJA, Simone JV, Pratt CB . Successful remission induction in children with acute lymphocytic leukemia at high risk for treatment failure Cancer 1971 27: 1332–1336
Sen L, Borella L . Clinical importance of lymphoblasts with T markers in childhood acute leukemia New Engl J Med 1975 292: 828–832
Secker-Walker LM, Lawler SD, Hardisty RM . Prognostic implications of chromosomal findings in acute lymphoblastic leukemia at diagnosis Br Med J 1978 2: 1529–1530
Williams DL, Tsiatis A, Brodeur GM, Look AT, Melvin SL, Bowman WP, Kalwinsky DK, Rivera G, Dahl GV . Prognostic importance of chromosome number in 136 untreated children with acute lymphoblastic leukemia Blood 1982 60: 864–871
Look AT, Roberson PK, Williams DL, Rivera G, Bowman WP, Pui CH, Ochs J, Abromowitch M, Kalwinsky D, Dahl GV . Prognostic importance of blast cell DNA content in childhood acute lymphoblastic leukemia Blood 1985 65: 1079–1086
Williams DL, Harder J, Murphy SB, Look AT, Kalwinsky DK, Rivera G, Melvin SL, Stass S, Dahl GV . Chromosomal translocations play a unique role in influencing prognosis in childhood acute lymphoblastic leukemia Blood 1986 68: 205–212
Pinkel D . Curing children of leukemia Cancer 1987 59: 1683–1691
Pinkel D . Species-specific therapy of acute lymphoid leukemia. In: Neth R, Gallo R (eds) Modern Trends In Human Leukemia VIII Springer-Verlag: New York 1989 27–35
Pinkel D . Genotypic classification of childhood acute lymphoid leukemia Leukemia 1999 13: S90–S91
Pinkel D, Woo S . Prevention and treatment of meningeal leukemia in children Blood 1994 84: 355–366
Mastrangelo R, Poplack D, Bleyer A, Riccardi R, Sather H, D'Angio G . Report and recommendations of the Rome workshop concerning poor-prognosis acute lymphoblastic leukemia in children: biologic bases for staging, stratification, and treatment Med Pediatr Oncol 1986 14: 191–194
Smith M, Arthur D, Camitta B, Carroll AJ, Crist W, Gaynon P, Gelber R, Heerema N, Korn EL, Link M, Murphy S, Pui CH, Pullen J, Reaman G, Sallan SE, Sather H, Shuster J, Simon R, Trigg M, Tubergen D, Uckun F, Ungerleider R . Uniform approach to risk classification and treatment assignment for children with acute lymphoblastic leukemia J Clin Oncol 1996 14: 18–24
Rubnitz JE, Behm FG, Pui CH, Evans WE, Relling MV, Raimondi SC, Harrison PL, Sandlunc JT, Ribeiro RC, Grosveld G, Downing JR . Genetic studies of childhood acute lymphoblastic leukemia with emphasis on p16, MLL, and ETV6 gene abnormalities: results of St Jude Total Therapy Study XII Leukemia 1997 11: 1201–1206
Hord MH, Smith TL, Culbert S, Frankel LS, Pinkel DP . Ethnicity and cure rates of Texas children with acute lymphoid leukemia Cancer 1996 77: 563–569
Bhatia S, Sather H, Zhang J, Trigg M, Gaynon P, Robinson L . Ethnicity and survival following childhood acute lymphoblastic leukemia (ALL): follow-up of the Children's Cancer Group (CCG) cohort Proc Am Soc Clin Oncol 1999 18: 568a (Abstr.)
Shuster J, Wacker P, Pullen J, Humbert J, Land VJ, Mahoney DH, Lauer S, Look AT, Borowitz MJ, Carroll AJ, Camitta B . Prognostic significance of sex in childhood B-precursor acute lymphoblastic leukemia: a Pediatric Oncology Group study J Clin Oncol 1998 16: 2854–2863
Pui CH, Boyett JM, Relling MV, Harrison PL, Rivera GK, Behm FG, Sandlund JT, Ribeiro RC, Rubnitz JE, Gajjar A, Evans WE . Sex differences in prognosis for children with acute lymphoblastic leukemia J Clin Oncol 1999 17: 818–824
Flandrin G, Brouet JC, Daniel MT, Preud'homme JL . Acute leukemia with Burkitt's tumor cells: a study of six cases with special references to lymphocyte surface markers Blood 1975 45: 183–188
Patte C, Philip T, Rodary C, Zucker JM, Behrandt H, Gentet JC, Lamagnere JP, Otten J, Dufillot D, Pein F, Caillou B, Lemerle J . High survival rate in advanced stage B cell lymphomas and leukemias without CNS involvement with a short intensive polychemotherapy: results from the French Pediatric Oncology Society of a randomized trial of 216 children J Clin Oncol 1991 9: 123–132
Pinkel D . Selecting treatment for children with acute lymphoblastic leukemia J Clin Oncol 1996 14: 4–6
Pui CH, Evans WE . Acute lymphoblastic leukemia. Drug therapy New Engl J Med 1998 339: 605–615
Kersey JH . Fifty years of studies of the biology and therapy of childhood leukemia Blood 1997 90: 4243–4251
Steinherz PG, Gaynon PS, Breneman JC, Cherlow FM, Grossman NJ, Kersey JH, Johnstone HS, Sather HN, Trigg ME, Chappell R, Hammond D, Bleyer WA . Cytoreduction and prognosis in acute lymphoblastic leukemia – the importance of early marrow response: report from the Childrens Cancer Group J Clin Oncol 1996 14: 389–398
Cavé H, Bosch JV, Suciu S, Guidal C, Waterkeyn C, Otten J, Bakkus M, Thielemans K, Grandchamp B, Vilmer E . Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia New Engl J Med 1998 339: 591–598
Pui CH, Boyett JM, Hancock ML, Pratt CB, Meyer WH, Crist WM . Outcome of treatment for childhood cancer in black as compared with white children JAMA 1995 273: 633–637
Grundy RG, Leiper AD, Stanhope R, Chessells JM . Survival and endocrine outcome after testicular relapse in acute lymphoblastic leukemia Arch Dis Child 1997 76: 190–196
Ritchey AK, Pollock BH, Lauer SJ, Andejeski Y, Buchanan GR . Improved survival of children with isolated CNS relapse of acute lymphoblastic leukemia: a Pediatric Oncology Group study J Clin Oncol 1999 17: 3745–3752
Billet AL, Pollock BH, Abshire TC, Buchanan GR . Outcome of children with B-lineage acute lymphoblastic leukemia in first bone marrow relapse Blood 1997 90: 332a (Abstr.)
Acknowledgements
Appreciation is expressed to the numerous collaborators in these studies: Drs T Zipf, S Lockhart, S Jeha, M Roberts, S Culbert, M Rytting, D Tubergen, the laboratory and nursing staffs of MD Anderson Cancer Center and Driscoll Children's Hospital, our secretary Mrs Irene Forbus, and particularly the children with leukemia and their parents who assented and consented to these studies.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Manera, R., Ramirez, I., Mullins, J. et al. Pilot studies of species-specific chemotherapy of childhood acute lymphoblastic leukemia using genotype and immunophenotype. Leukemia 14, 1354–1361 (2000). https://doi.org/10.1038/sj.leu.2401835
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2401835
Keywords
This article is cited by
-
Remembering Donald Pinkel, MD: Acute lymphoblastic leukaemia pioneer September 7, 1926 –March 9, 2022
Leukemia (2022)
-
‘Allogeneic marrow transplantation in children with acute leukemia: a practice whose time has gone’: twenty years later
Leukemia (2009)
-
Treatment by design in leukemia, a meeting report, Philadelphia, Pennsylvania, December 2002
Leukemia (2003)
-
Benefit of high-dose methylprednisolone in comparison with conventional-dose prednisolone during remission induction therapy in childhood acute lymphoblastic leukemia for long-term follow-up
Leukemia (2003)
-
BFM-oriented treatment for children with acute lymphoblastic leukemia without cranial irradiation and treatment reduction for standard risk patients: results of DCLSG protocol ALL-8 (1991–1996)
Leukemia (2002)