Abstract
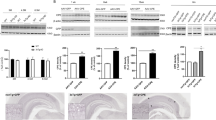
Insulin-like growth factor-I (IGF-I) is emerging as a powerful neuroprotective molecule that is strongly induced in the central nervous system after different insults. We constructed a recombinant adenoviral vector (RAd-IGFI) harboring the gene for rat IGF-I and used it to implement IGF-I gene therapy in the hypothalamus of senile female rats, which display hypothalamic dopaminergic (DA) neurodegeneration and as a consequence, chronic hyperprolactinemia. Restorative IGF-I gene therapy was implemented in young (5 months) and senile (28 months) female rats, which received a single intrahypothalamic injection of 3 × 109 plaque-forming units of RAd-βgal (a control adenoviral vector expressing β-galactosidase) or RAd-IGFI and were killed 17 days post-injection. In the young animals, neither vector modified serum prolactin levels, but in the RAd-IGFI-injected senile rats a nearly full reversion of their hyperprolactinemic status was recorded. Morphometric analysis revealed a significant increase in the total number of tyrosine hydroxylase-positive cells in the hypothalamus of experimental as compared with control senile animals (5874±486 and 3390±498, respectively). Our results indicate that IGF-I gene therapy in senile female rats is highly effective for restoring their hypothalamic DA dysfunction and thus reversing their chronic hyperprolactinemia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Korell M, Tanner CM . Epidemiology of Parkinson's disease: an overview. In: Ebadi M, Pfeiffer RF (eds). Parkinson's Disease. CRC Press: Boca Raton, FL, 2004, pp 233–242.
Sarkar DK, Gottschall PE, Meites J . Damage to hypothalamic dopaminergic neurons is associated with development of prolactin-secreting pituitary tumors. Science 1982; 218: 684–686.
Goya RG, Lu JKH, Meites J . Gonadal function and pituitary and mammary pathology in the aging rat. Mech Age Devel 1990; 56: 77–88.
Cónsole G, Gómez Dumm CL, Brown OA, Goya RG . Sexual dimorphism in the age changes of the pituitary lactotrophs in rats. Mech Age Devel 1997; 95: 157–166.
Franceschi M, Camerlingo M, Perego L, Bottacchi E, Mamoli A . Tuberoinfundibular dopaminergic function in Parkinson's disease. Eur Neurol 1988; 28: 117–119.
Beilharz EJ, Russo VC, Butler G, Baker NL, Connor B, Sirimanne ES et al. Co-ordinated and cellular specific induction of the components of the IGF/IGFBP axis in the rat brain following hypoxic–ischemic injury. Brain Res Mol Brain Res 1998; 59: 119–134.
Li XS, Williams M, Bartlett WP . Induction of IGF-1 mRNA expression following traumatic injury to the postnatal brain. Brain Res Mol Brain Res 1998; 57: 92–96.
Walter HJ, Berry M, Hill DJ, Logan A . Spatial and temporal changes in the insulin-like growth factor (IGF) axis indicate autocrine/paracrine actions of within wounds of the rat brain. Endocrinology 1997; 138: 3024–3034.
Yao DL, West NR, Bondy CA, Brenner M, Hudson LD, Zhou J et al. Cryogenic spinal cord injury induces astrocytic gene expression of insulin-like growth factor I and insulin-like growth factor binding protein 2 during myelin regeneration. J Neurosci Res 1995; 40: 647–659.
Breese CR, D'Costa A, Rollins YD, Adams C, Booze RM, Sonntag WE et al. Expression of insulin-like growth factor-1 (IGF-I) and IGF-binding protein 2(IGF-BP2) in the hippocampus following cytotoxic lesion of the dentate gyrus. J Comp Neurol 1996; 369: 388–404.
Dore S, Kar S, Quirion R . Insulin-like growth factor-1 protects and rescues hippocampal neurons against amyloid- and amylin-induced toxicity. Proc Natl Acad Sci USA 1997; 94: 4772–4777.
Carro E, Trejo JL, Gomez-Isla T, LeRoith D, Torres-Aleman I . Serum insulin-like growth factor I regulates brain amyloid-beta levels. Nat Med 2002; 8: 1390–1397.
Torres-Aleman I, Naftolin F, Robbins RJ . Trophic effects of insulin-like growth factor-1 on fetal rat hypothalamic cells in culture. Neuroscience 1990; 35: 601–608.
Knusel B, Michel PP, Schwaber JS, Hefti F . Selective and nonselective stimulation of central cholinergic and dopaminergic development in vitro by nerve growth factor, basic fibroblast growth factor, epidermal growth factor, insulin and the insulin-like growth factors I and II. J Neurosci 1990; 10: 558–570.
Sortino MA, Canonico PL . Neuroprotective effect of insulin-like growth factor I in immortalized hypothalamic cells. Endocrinology 1996; 137: 1418–1422.
Shavali S, Ren J, Ebadi M . Insulin-like growth factor-1 protects human dopaminergic SH-SY5Y cells from salsolinol-induced toxicity. Neurosci Lett 2003; 340: 79–82.
Offen D, Shtaif B, Hadad D, Weizman A, Melamed E, Gil-Ad I . Protective effect of insulin-like-growth-factor-1 against dopamine-induced neurotoxicity in human and rodent neuronal cultures: possible implications for Parkinson's disease. Neurosci Lett 2001; 316: 129–132.
Shimohama S, Sawada H, Kitamura Y, Taniguchi T . Disease model: Parkinson's disease. Trends Mol Med 2003; 9: 360–365.
de Rijk MC, Breteler MM, Graveland GA, Ott A, Grobbee DE, van der Meche FG et al. Prevalence of Parkinson's disease in the elderly: the Rotterdam study. Neurology 1995; 45: 2143–2146.
Mayeux R, Marder K, Cote LJ, Denaro J, Hemenegildo N, Mejia H et al. The frequency of idiopathic Parkinson's disease by age, ethnic group and sex in northern Manhattan, 1988–1993. Am J Epidemiol 1995; 142: 820–827.
Valtin H . Genetic models of diabetes insipidus. In: Windhager EE (ed). Handbook of Physiology. Oxford University Press: New York, 1992, pp 1281–1316.
Geddes BJ, Harding TC, Lightman SL, Uney JB . Long-term gene therapy in the CNS: reversal of hypothalamic diabetes insipidus in the Brattleboro rat by using an adenovirus expressing arginine vasopressin. Nature Med 1997; 3: 1402–1404.
Dahlström A, Fuxe K . Evidence for the existence of monoamine containing neurons in the central nervous system. I. Demonstration of monoamines in the cell bodies of brain stem neurons. Acta Physiol Scand 1964; 62: 1–55.
Tillet Y, Kitahama K . Distribution of central catecholaminergic neurons: a comparison between ungulates, humans and other species. Histol Histopathol 1998; 13: 1163–1177.
Kawano H, Daikoku S . Functional topography of the rat hypothalamic dopamine neuron systems: retrograde tracing and immunohistochemical study. J Comp Neurol 1997; 265: 242–253.
Goudreau JL, Lindley SE, Lookingland KJ, Moore KE . Evidence that hypothalamic periventricular dopamine neurons innervate the intermediate lobe of the rat pituitary. Neuroendocrinology 1992; 40: 145–151.
Ben-Jonathan N, Arbogast LA, Hyde JF . Neuroendocrine regulation of prolactin release. Prog Neurobiol 1989; 33: 399–477.
Porter JC, Nansel DD, Gudelsky GA, Reymond MJ, Pilotte NS, Foreman MM et al. Some aspects of hypothalamic and hypophysial secretion in aging rats. Peptides 1980; 1: 135–139.
Reymond MJ, Porter JC . Secretion of hypothalamic dopamine into pituitary stalk blood of aged female rats. Brain Res Bull 1981; 7: 69–73.
Gudelsky GA, Nansel DD, Porter JC . Dopaminergic control of prolactin secretion in the aging male rat. Brain Res 1981; 204: 446–450.
Porter JC, Aguila-Mansilla N, Ramin SM, Kedzierski W . Secretion by hypothalamic dopaminergic neurons of the aged brain. Neurobiol Aging 1994; 15: 535–539.
Sánchez HL, Silva LB, Portiansky EL, Goya RG, Zuccolilli GO . Impact of very old age on hypothalamic dopaminergic neurons in the female rat: A morphometric study. J Comp Neurol 2003; 458: 319–325.
Fernandez AM, de la Vega AG, Torres-Aleman I . Insulin-like growth factor I restores motor coordination in a rat model of cerebellar ataxia. Proc Natl Acad Sci USA 1998; 95: 1253–1258.
Markowska AL, Mooney M, Sonntag WE . Insulin-like growth factor-1 meliorates age-related behavioral deficits. Neuroscience 1998; 87: 559–569.
Carro E, Trejo JL, Busiguina S, Torres-Aleman I . Circulating insulin-like growth factor I mediates the protective effects of physical exercise against brain insults of different etiology and anatomy. J Neurosci 2001; 21: 5678–5684.
Aberg MA, Aberg ND, Hedbacker H, Oscarsson J, Eriksson PS . Peripheral infusion of IGF-I selectively induces neurogenesis in the adult rat hippocampus. J Neurosci 2000; 20: 2896–2903.
Trejo JL, Carro E, Torres-Aleman I . Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J Neurosci 2001; 21: 1628–1634.
Xu Y, Tamamaki N, Noda T, Kimura K, Itokazu Y, Matsumoto N et al. Neurogenesis in the ependymal layer of the adult rat 3rd ventricle. Exp Neurol 2005; 192: 251–264.
Hitt M, Bett A, Prevec L, Graham FL . Construction and propagation of human adenovirus vectors. In: JE Celis (ed). Cell Biology: A Laboratory Handbook. Academic Press: San Diego, CA, 1998, pp 1500–1512.
Daughaday WH, Rotwein P . Insulin-like growth factors I and II: peptide, messenger ribonucleic acid and gene structures, serum and tissue concentrations. Endocr Rev 1989; 10: 68–91.
Paxinos G, Watson C . The Rat Brain in Stereotaxic Coordinates. Academic Press: San Diego, 1998.
Breier BH, Gallaher BW, Gluckman PD . Radioimmunoassay for insulin-like growth factor-I: solutions to some potential problems and pitfalls. J Endocrinol 1991; 128: 347–357.
Diaz-Torga G, Feierstein C, Libertun C, Gelman D, Kelly MA, Low MJ et al. Disruption of the D2 dopamine receptor alters GH and IGF-I secretion and causes dwarfism in male mice. Endocrinology 2002; 43: 1270–1279.
Acknowledgements
This work was supported in part by Grants # PICT13588 and PICT10663 from the (Argentine) National Agency for the Promotion of Science and Technology and Grant NIH#1 R21 TW006665 from the Fogarty International Center and the National Institute on Aging (USA) to RGG, and Grant # PICT 12544 to ELP. We thank Ms Yolanda Sosa for technical assistance and Ms Paula Reggiani for graphic design assistance. OJR, DBV, ELP and RGG are Research Career scientists of the Argentine Research Council (CONICET). CBH and CC are doctoral fellowship recipients from CONICET.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hereñú, C., Cristina, C., Rimoldi, O. et al. Restorative effect of insulin-like growth factor-I gene therapy in the hypothalamus of senile rats with dopaminergic dysfunction. Gene Ther 14, 237–245 (2007). https://doi.org/10.1038/sj.gt.3302870
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302870
Keywords
This article is cited by
-
IGF-1 Gene Transfer Modifies Inflammatory Environment and Gene Expression in the Caudate-Putamen of Aged Female Rat Brain
Molecular Neurobiology (2022)
-
IGF1 Gene Therapy Reversed Cognitive Deficits and Restored Hippocampal Alterations After Chronic Spinal Cord Injury
Molecular Neurobiology (2021)
-
Adenovirus-Mediated Transduction of Insulin-Like Growth Factor 1 Protects Hippocampal Neurons from the Toxicity of Aβ Oligomers and Prevents Memory Loss in an Alzheimer Mouse Model
Molecular Neurobiology (2020)
-
Regulatable adenovector harboring the GFP and Yamanaka genes for implementing regenerative medicine in the brain
Gene Therapy (2019)
-
Umbilical Cord Cell Therapy Improves Spatial Memory in Aging Rats
Stem Cell Reviews and Reports (2019)