Abstract
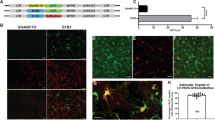
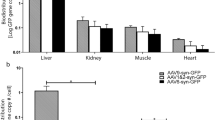
The development of efficient systems for in vivo gene transfer to the central nervous system (CNS) may provide a useful therapeutic strategy for the alleviation of several neurological disorders. In this study, we evaluated the feasibility of nonviral gene therapy to the CNS mediated by cationic liposomes. We present evidence of the successful delivery and expression of both a reporter and a therapeutic gene in the rodent brain, as evaluated by immunohistochemical assays. Our results indicate that transferrin-associated cationic liposome/DNA complexes (Tf-lipoplexes) allow a significant enhancement of transfection activity as compared to plain complexes, and that 8/1 (+/−) Tf-lipoplexes constitute the best formulation to mediate in vivo gene transfer. We demonstrated that Tf-lipoplex-mediated nerve growth factor transgene expression attenuates the morphological damages of the kainic acid-induced lesion as assessed by 2,3,5-triphenyltetrazolium chloride (TTC) vital staining. These findings suggest the usefulness of these lipid-based vectors in mediating the delivery of therapeutic genes to the CNS.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Pardridge WM . CNS drug design based on principles of blood–brain barrier transport. J Neurochem 1998; 70: 1781–1792.
Cornford EM, Hyman S . Blood–brain barrier permeability to small and large molecules. Adv Drug Deliv Rev 1999; 36: 145–163.
Zhang Y et al. Normalization of striatal tyrosine hydroxylase and reversal of motor impairment in experimental parkinsonism with intravenous nonviral gene therapy and a brain-specific promoter. Hum Gene Ther 2004; 15: 339–350.
Zhang Y, Zhu C, Pardridge WM . Antisense gene therapy of brain cancer with an artificial virus gene delivery system. Mol Ther 2002; 6: 67–72.
Blesch A, Uy HS, Diergardt N, Tuszynski MH . Neurite outgrowth can be modulated in vitro using a tetracycline-repressible gene therapy vector expressing human nerve growth factor. J Neurosci Res 2000; 59: 402–409.
Martinez-Serrano A, Olsson M, Gates MA, Bjorklund A . In utero gene transfer reveals survival effects of nerve growth factor on rat brain cholinergic neurons during development. Eur J Neurosci 1998; 10: 263–271.
Wu D, Pardridge WM . Neuroprotection with noninvasive neurotrophin delivery to the brain. Proc Natl Acad Sci USA 1999; 96: 254–259.
Martinez-Serrano A, Hantzopoulos PA, Bjorklund A . Ex vivo gene transfer of brain-derived neurotrophic factor to the intact rat forebrain – neurotrophic effects on cholinergic neurons. Eur J Neurosci 1996; 8: 727–735.
Tenenbaum L et al. Neuroprotective gene therapy for Parkinson's disease. Curr Gene Ther 2002; 2: 451–483.
Klein RL et al. Neuron-specific transduction in the rat septohippocampal or nigrostriatal pathway by recombinant adeno-associated virus vectors. Exp Neurol 1998; 150: 183–194.
Tsai TH et al. Recombinant adeno-associated virus vector expressing glial cell line-derived neurotrophic factor reduces ischemia-induced damage. Exp Neurol 2000; 166: 266–275.
Caleo M et al. Expression of BCL-2 via adeno-associated virus vectors rescues thalamic neurons after visual cortex lesion in the adult rat. Eur J Neurosci 2002; 15: 1271–1277.
Buchet D et al. Long-term expression of beta-glucuronidase by genetically modified human neural progenitor cells grafted into the mouse central nervous system. Mol Cell Neurosci 2002; 19: 389–401.
D'Costa J et al. HIV-2 derived lentiviral vectors: gene transfer in Parkinson's and Fabry disease models in vitro. J Med Virol 2003; 71: 173–182.
Jakobsson J et al. Targeted transgene expression in rat brain using lentiviral vectors. J Neurosci Res 2003; 73: 876–885.
de Almeida LP et al. Lentiviral-mediated delivery of mutant huntingtin in the striatum of rats induces a selective neuropathology modulated by polyglutamine repeat size, huntingtin expression levels, and protein length. J Neurosci 2002; 22: 3473–3483.
Blomer U et al. Highly efficient and sustained gene transfer in adult neurons with a lentivirus vector. J Virol 1997; 71: 6641–6649.
Naldini L et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 1996; 272: 263–267.
Zou LL et al. Liposome-mediated NGF gene transfection following neuronal injury: potential therapeutic applications. Gene Therapy 1999; 6: 994–1005.
Shi N et al. Brain-specific expression of an exogenous gene after i.v. administration. Proc Natl Acad Sci USA 2001; 98: 12754–12759.
Wattiaux R et al. Cationic lipids delay the transfer of plasmid DNA to lysosomes. Biochem Biophys Res Commun 1996; 227: 448–454.
Khaw BA et al. Intracytoplasmic gene delivery for in vitro transfection with cytoskeleton-specific immunoliposomes. J Control Release 2001; 75: 199–210.
Li S et al. Dynamic changes in the characteristics of cationic lipidic vectors after exposure to mouse serum: implications for intravenous lipofection. Gene Therapy 1999; 6: 585–594.
Sakurai F et al. Interaction between DNA-cationic liposome complexes and erythrocytes is an important factor in systemic gene transfer via the intravenous route in mice: the role of the neutral helper lipid. Gene Therapy 2001; 8: 677–686.
Liu Y et al. Factors influencing the efficiency of cationic liposome-mediated intravenous gene delivery. Nat Biotech 1997; 15: 167–173.
Brooks AI et al. Reproducible and efficient murine CNS gene delivery using a microprocessor-controlled injector. J Neurosci Methods 1998; 80: 137–147.
Imaoka T et al. In vivo gene transfer into the adult mammalian central nervous system by continuous injection of plasmid DNA-cationic liposome complex. Brain Res 1998; 780: 119–128.
Li Y et al. CNS gene transfer mediated by a novel controlled release system based on DNA complexes of degradable polycation PPE-EA: a comparison with polyethylenimine/DNA complexes. Gene Therapy 2004; 11: 109–114.
Imaoka T, Date I, Ohmoto T, Nagatsu T . Significant behavioral recovery in Parkinson's disease model by direct intracerebral gene transfer using continuous injection of a plasmid DNA-liposome complex. Hum Gene Ther 1998; 9: 1093–1102.
Ong WY et al. Changes in glutathione in the hippocampus of rats injected with kainate: depletion in neurons and upregulation in glia. Exp Brain Res 2000; 132: 510–516.
Ferraguti F et al. Activated astrocytes in areas of kainate-induced neuronal injury upregulate the expression of the metabotropic glutamate receptors 2/3 and 5. Exp Brain Res 2001; 137: 1–11.
Alberch J, Perez-Navarro E, Canals JM . Neuroprotection by neurotrophins and GDNF family members in the excitotoxic model of Huntington's disease. Brain Res Bull 2002; 57: 817–822.
Liu HM, Lei DL, Yang DL . Kainate-induced brain lesion: similar local and remote histopathological and molecular changes as in ischemic brain infarct. J Neuropathol Exp Neurol 1996; 55: 787–794.
de Lima MCP et al. Gene delivery mediated by cationic liposomes: from biophysical aspects to enhancement of transfection. Mol Membr Biol 1999; 16: 103–109.
Simoes S et al. Gene delivery by negatively charged ternary complexes of DNA, cationic liposomes and transferrin or fusigenic peptides. Gene Therapy 1998; 5: 955–964.
Simoes S et al. Successful transfection of lymphocytes by ternary lipoplexes. Biosci Rep 1999; 19: 601–609.
Simoes S et al. Mechanisms of gene transfer mediated by lipoplexes associated with targeting ligands or pH-sensitive peptides. Gene Therapy 1999; 6: 1798–1807.
da Cruz MT, Simoes S, de Lima MC . Improving lipoplex-mediated gene transfer into C6 glioma cells and primary neurons. Exp Neurol 2004; 187: 65–75.
Lin MT, Wang F, Uitto J, Yoon K . Differential expression of tissue-specific promoters by gene gun. Br J Dermatol 2001; 144: 34–39.
Douglas JL, Lin WY, Panis ML, Veres G . Efficient human immunodeficiency virus-based vector transduction of unstimulated human mobilized peripheral blood CD34+ cells in the SCID-hu Thy/Liv model of human T cell lymphopoiesis. Hum Gene Ther 2001; 12: 401–413.
de Almeida LP, Zala D, Aebischer P, Deglon N . Neuroprotective effect of a CNTF-expressing lentiviral vector in the quinolinic acid rat model of Huntington's disease. Neurobiol Dis 2001; 8: 433–446.
Butcher SP, Bullock R, Graham DI, McCulloch J . Correlation between amino acid release and neuropathologic outcome in rat brain following middle cerebral artery occlusion. Stroke 1990; 21: 1727–1733.
Massieu L, Gomez-Roman N, Montiel T . In vivo potentiation of glutamate-mediated neuronal damage after chronic administration of the glycolysis inhibitor iodoacetate. Exp Neurol 2000; 165: 257–267.
Obrenovitch TP, Urenjak J . Altered glutamatergic transmission in neurological disorders: from high extracellular glutamate to excessive synaptic efficacy. Prog Neurobiol 1997; 51: 39–87.
Kristian T, Siesjo BK . Calcium in ischemic cell death. Stroke 1998; 29: 705–718.
Limbrick Jr DD, Pal S, DeLorenzo RJ . Hippocampal neurons exhibit both persistent Ca2+ influx and impairment of Ca2+ sequestration/extrusion mechanisms following excitotoxic glutamate exposure. Brain Res 2001; 894: 56–67.
Brorson JR, Manzolillo PA, Miller RJ . Ca2+ entry via AMPA/KA receptors and excitotoxicity in cultured cerebellar Purkinje cells. J Neurosci 1994; 14: 187–197.
Ferreira IL, Duarte CB, Carvalho AP . Ca2+ influx through glutamate receptor-associated channels in retina cells correlates with neuronal cell death. Eur J Pharmacol 1996; 302: 153–162.
Santos AE, Carvalho AL, Lopes MC, Carvalho AP . Differential postreceptor signaling events triggered by excitotoxic stimulation of different ionotropic glutamate receptors in retinal neurons. J Neurosci Res 2001; 66: 643–655.
McLaughlin J et al. Sparing of neuronal function postseizure with gene therapy. Proc Natl Acad Sci USA 2000; 97: 12804–12809.
Widenfalk J et al. Neurotrophic factors and receptors in the immature and adult spinal cord after mechanical injury or kainic acid. J Neurosci 2001; 21: 3457–3475.
Shinoura N et al. Adenovirus-mediated transfer of caspase-8 augments cell death in gliomas: implication for gene therapy. Hum Gene Ther 2000; 11: 1123–1137.
Elliger SS et al. Elimination of lysosomal storage in brains of MPS VII mice treated by intrathecal administration of an adeno-associated virus vector. Gene Therapy 1999; 6: 1175–1178.
Yoshida J et al. Human gene therapy for malignant gliomas (glioblastoma multiforme and anaplastic astrocytoma) by in vivo transduction with human interferon beta gene using cationic liposomes. Hum Gene Ther 2004; 15: 77–86.
Janson C et al. Clinical protocol Gene therapy of Canavan disease: AAV-2 vector for neurosurgical delivery of aspartoacylase gene (ASPA) to the human brain. Hum Gene Ther 2002; 13: 1391–1412.
Mok KWC, Cullis PR . Structural and fusogenic properties of cationic liposomes in the presence of plasmid DNA. Biophys J 1997; 73: 2534–2545.
Thorne RG, Frey WH . Delivery of neurotrophic factors to the central nervous system: pharmacokinetic considerations. Clin Pharmacokinet 2001; 40: 907–946.
Martinez-Serrano A, Bjorklund A . Ex vivo nerve growth factor gene transfer to the basal forebrain in presymptomatic middle-aged rats prevents the development of cholinergic neuron atrophy and cognitive impairment during aging. Proc Natl Acad Sci USA 1998; 95: 1858–1863.
Chen KS, Gage FH . Somatic gene transfer of NGF to the aged brain – Behavioral and morphological amelioration. J Neurosci 1995; 15: 2819–2825.
Gouhier C et al. Neuroprotection of nerve growth factor-loaded microspheres on the D2 dopaminergic receptor positive-striatal neurones in quinolinic acid-lesioned rats: a quantitative autoradiographic assessment with iodobenzamide. Neurosci Lett 2000; 288: 71–75.
Martinez-Serrano A, Bjorklund A . Protection of the neostriatum against excitotoxic damage by neurotrophin-producing, genetically modified neural stem cells. J Neurosci 1996; 16: 4604–4616.
Perez-Navarro E, Alberch J . Protective role of nerve growth factor against excitatory amino acid injury during neostriatal cholinergic neurons postnatal development. Exp Neurol 1995; 135: 146–152.
Paxinos G, Watson C . The Rat Brain in Stereotaxic Coordinates. Academic Press Inc.: San Diego, 1986.
Lin TN et al. Effect of brain edema on infarct volume in a focal cerebral ischemia model in rats. Stroke 1993; 24: 117–121.
Acknowledgements
We thank Professor Patrick Aebischer, Institut des Neurosciences, École Polytechnique de Lausanne, Lausanne, Switzerland, for providing the pSIN-PGK-nls-LacZ-WHV plasmid, and Dr Robert Debs, California Pacific Medical Center Research Institute, San Francisco, USA, for the pCMV-NGF plasmid generous gift. We are grateful to Professor Tice Macedo and to Frederico Pereira for providing access to the stereotactic frame used throughout this study. M Teresa Girão da Cruz was the recipient of a fellowship from the Portuguese Foundation for Science and Technology (PRAXIS XXI/BD/19529/99). This work was partially financed by a grant from the Portuguese Foundation for Science and Technology (FCT/BIO/36202/00).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Teresa Girão da Cruz, M., Cardoso, A., de Almeida, L. et al. Tf-lipoplex-mediated NGF gene transfer to the CNS: neuronal protection and recovery in an excitotoxic model of brain injury. Gene Ther 12, 1242–1252 (2005). https://doi.org/10.1038/sj.gt.3302516
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302516
Keywords
This article is cited by
-
CNS Delivery of Nucleic Acid Therapeutics: Beyond the Blood–Brain Barrier and Towards Specific Cellular Targeting
Pharmaceutical Research (2023)
-
No effects of power line frequency extremely low frequency electromagnetic field exposure on selected neurobehavior tests of workers inspecting transformers and distribution line stations versus controls
Australasian Physical & Engineering Sciences in Medicine (2014)
-
Transferrin lipoplex-mediated suicide gene therapy of oral squamous cell carcinoma in an immunocompetent murine model and mechanisms involved in the antitumoral response
Cancer Gene Therapy (2009)
-
Transferrin-Associated Lipoplexes as Gene Delivery Systems: Relevance of Mode of Preparation and Biophysical Properties
Journal of Membrane Biology (2008)
-
Nonviral Approaches for Neuronal Delivery of Nucleic Acids
Pharmaceutical Research (2008)