Abstract
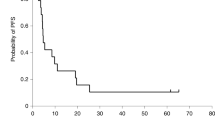
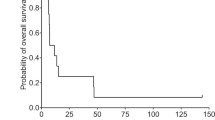
Following standard treatment, the prognosis remains poor in patients with high-grade glioma and new therapies are urgently required. Herpes simplex virus 1716 (HSV1716) is an ICP34.5 null mutant that is selectively replication competent and shown to be safe and to replicate following injection into high-grade glioma. We demonstrate that following surgical resection, HSV1716 is safe when injected into the brain adjacent to excised tumour. In all, 12 patients with recurrent or newly diagnosed high-grade glioma underwent maximal resection of the tumour. HSV1716 was injected into eight to 10 sites around the resulting tumour cavity with the intent of infecting residual tumour cells. As clinically indicated, patients proceeded to further radiotherapy or chemotherapy. There has been no clinical evidence of toxicity associated with the administration of HSV1716. Longitudinal follow-up has allowed the assessment of overall survival compared to that of similar patients not treated with HSV1716. Three patients remain alive and clinically stable at 15, 18 and 22 months postsurgery and HSV1716 injection. Remarkably, the first patient in the trial, who had extensive recurrent disease preprocedure, is alive at 22 months since injection of HSV1716 and 29 months since first diagnosis. Imaging has demonstrated a reduction of residual tumour over the 22-month period despite no further medical intervention since the surgery and HSV1716 injection. In this study, we demonstrate that on the basis of clinical observations, there has been no toxicity following the administration of HSV1716 into the resection cavity rim in patients with high-grade glioma. The survival and imaging data, in addition to the lack of toxicity, give us confidence to proceed to a clinical trial to demonstrate efficacy of HSV1716 in glioma patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Rampling R et al. Toxicity evaluation of replication-competent herpes simplex virus (ICP 34.5 null mutant 1716) in patients with recurrent malignant glioma. Gene Therapy 2000; 7: 859–866.
Papanastassiou V et al. The potential for efficacy of the modified (ICP34.5−) herpes simplex virus HSV1716 following intra tumoural injection into human malignant glioma: a proof of principle study. Gene Therapy 2002; 9: 398–406.
Dolan A, McKie E, MacLean A, McGeoch D . Status of ICP34.5 gene in herpes simplex virus type 1 strain 17. J Gen Virol 1992; 73: 971–973.
Ackermann M et al. Identification of a protein specified by a diploid gene located in the terminal repeats of the L component of the herpes simplex virus genome. J Virol 1986; 58: 843–850.
Brown S M et al. Cell type and cell state determine differential in vitro growth of non virulent ICP34.5 negative herpes simplex virus type 1 mutant. J Gen Virol 1994; 75: 2367–2377.
McKie EA et al. Selective in vitro killing of primary CNS tumour using herpes simplex virus type 1 (HSV-1), ICP345 null mutants – a potentially effective clinical therapy. Br J Cancer 1996; 74: 745–752.
Kesari S et al. Therapy of experimental human brain tumours using a neuroattenuated herpes simplex virus mutant. Lab Invest 1995; 73: 636–648.
MacLean AR et al. Herpes simplex virus type 1 deletion variants 1714 and 1716 pinpoint neurovirulence-related sequences in Glasgow strain 17+ between immediate early gene1 and the ‘a’ sequence. J Gen Virol 1991; 72: 631–639.
Harland J, Brown SM . HSV growth preparation and assay. In: Brown SM, MacLean AR (eds) Herpes Simplex Virus Protocols. Methods in Molecular Medicine. Humana Press: Totowa, NJ, 1997.
Arbuck SG . Workshop on phase 1 study design. Ninth NCI/EORTC New Drug Development Symposium, Amsterdam, March 1996. Ann Oncol 1996; 7: 567–573.
Steward WS . Phase 1 studies-alternative endpoints: when is toxicity not appropriate? In Ninth NCI/EORTC Symposium on New Drugs in Cancer Therapy, Amsterdam 1996.
Markert JM, Medlock MD, Rabkin SD et al. Conditionally replicating herpes simplex virus mutant G207 for the treatment of malignant glioma: results of a phase 1 trial. Gene Therapy 2000; 7: 867–874.
Harland J, Papanastassiou V, Brown S M . HSV1716 persistence in primary human glioma cells in vitro. Gene Therapy 2002; 9: 1194–1198.
Rajan B et al. Survival in patients with recurrent glioma as a measure of treatment efficacy: prognostic factors following nitrosourea chemotherapy. Eur J Cancer 1994; 30A: 1809–1815.
Acknowledgements
This work was supported by a grant from the Biomedical and Therapeutics Research Committee (BTRC) of the Scottish Executive, Department of Health. We greatly appreciate their continued support in the clinical programme for HSV1716 in cancer.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Harrow, S., Papanastassiou, V., Harland, J. et al. HSV1716 injection into the brain adjacent to tumour following surgical resection of high-grade glioma: safety data and long-term survival. Gene Ther 11, 1648–1658 (2004). https://doi.org/10.1038/sj.gt.3302289
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302289
Keywords
This article is cited by
-
Clinical advances in oncolytic virus therapy for malignant glioma: a systematic review
Discover Oncology (2023)
-
Clinical trial links oncolytic immunoactivation to survival in glioblastoma
Nature (2023)
-
Oncolytic Viral Therapy for Malignant Glioma and Their Application in Clinical Practice
Neurotherapeutics (2022)
-
Implications of immune cells in oncolytic herpes simplex virotherapy for glioma
Brain Tumor Pathology (2022)
-
Glioblastoma infiltration of both tumor- and virus-antigen specific cytotoxic T cells correlates with experimental virotherapy responses
Scientific Reports (2020)