Abstract
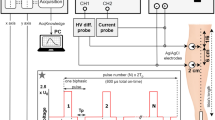
Heat-sensitive transgene expression systems have been proposed recently for use in gene therapy to enable both spatial and temporal control of the gene activity. The transgene was put under the control of HSP-related promoters and could be turned on by external heat treatment. While the ‘heat activation’ phenomenon of the HSP-related promoters in vitro had been well documented, the detailed time response and temporal regulation profile in vivo were not fully understood. We reported here the regulation of transgene luciferase expression in vivo in muscles using a custom-built ultrasound-mediated hyperthermia instrument. The effects of different heating parameters and treatment regimens were evaluated. Optimal activation of gene expression was found at 39°C. Significant tissue damage was observed at 41°C and above, which directly correlated with the greatly reduced gene expression. The gene constructs remained stable and silent in muscle cells, and could be turned on at a later time without losing much activity. Repeated activation was also possible, but required heat treatment at a higher temperature to overcome thermotolerance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Gossen M, Bujard H . Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA 1992; 89: 5547–5551.
Magari SR et al. Pharmacologic control of a humanized gene therapy system implanted into nude mice. J Clin Invest 1997; 100: 2865–2872.
Gossen M et al. Transcriptional activation by tetracyclines in mammalian cells. Science 1995; 268: 1766–1769.
Deuschle D, Meyer WK-H, Thiesen H-J . Tetracycline-reversible silencing of eukaryotic promoters. Mol Cell Biol 1995; 15: 1907–1914.
Sturtz FG et al. Tetracycline-regulatable expression vectors tightly regulate in vitro gene expression of secreted proteins. Gene 1998; 221: 279–285.
Rizzuto G et al. Efficient and regulated erythropoietin production by naked DNA injection and muscle electroporation. Proc Natl Acad Sci USA 1999; 96: 6417–6422.
Perez N et al. Tetracycline transcriptional silencer tightly controls transgene expression after in vivo intramuscular electrotransfer: application to interleukin 10 therapy in experimental arthritis. Hum Gene Ther 2002; 13: 2161–2172.
Harrington KJ, Linardakis E, Vile RG . Transcriptional control: an essential component of cancer gene therapy strategies? Adv Drug Deliv Rev 2000; 44: 167–184.
Steiner MS et al. In vivo expression of prostate-specific adenoviral vectors in a canine model. Cancer Gene Ther 1999; 6: 456–464.
Weichselbaum RR et al. Radiation-induced tumour necrosis factor-α expression: clinical application of transcriptional and physical targeting of gene therapy. Lancet Oncol 2002; 3: 665–671.
Datta R et al. Ionizing radiation activates transcription of the EGR1 via CarG elements. Proc Natl Acad Sci USA 1992; 89: 10149–10153.
Greco O et al. Novel chimeric gene promoters responsive to hypoxia and ionizing radiation. Gene Therapy 2002; 9: 1403–1411.
Li CY, Dewhirst MW . Hyperthermia-regulated immunogene therapy. Int J Hyperthermia 2002; 18: 586–596.
Smith RC et al. Spatial and temporal control of transgene expression through ultrasound-mediated induction of the heat shock protein 70B promoter in vivo. Hum Gene Ther 2002; 13: 697–706.
Song J, Xu LX, Lemons DE, Weinbaum S . Microvascular thermal equilibration in rat spinotrapezius muscle. Ann Biomed Eng 1999; 27: 56–66.
Wust P et al. Hyperthermia in combined treatment of cancer. Lancet Oncol 2002; 3: 487–497.
Guilhon E et al. Spatial and temporal control of transgene expression in vivo using a heat-sensitive promoter and MRI-guided focused ultrasound. J Gene Med 2003; 5: 333–342.
Aihara H, Miyazaki J . Gene transfer into muscle by electroporation in vivo. Nat Biotechnol 1998; 16: 867–870.
Vekris A et al. Control of transgene expression using local hyperthermia in combination with a heat sensitive promoter J. Gene Med 2000; 2: 89–96.
Guilhon E et al. Image-guided control of transgene expression based on local hyperthermia. Mol Imaging 2003; 2: 11–17.
Huang Q et al. Heat-induced gene expression as a novel targeted cancer gene therapy strategy. Cancer Res 2000; 60: 3435–3439.
Li GC, Mivechi NF, Weitzel G . Heat shock proteins, thermotolerance, and their relevance to clinical hyperthermia. Int J Hyperthermia 1995; 11: 459–488.
Hildebrandt B et al. The cellular and molecular basis of hyperthermia. Crit Rev Oncol/Hematol 2002; 43: 33–56.
Xu YH, Hui SW, Frederik P, Szoka FC . Physicochemical characterization and purification of cationic lipoplexes. Biophys J 1999; 77: 341–353.
Hamer PW, McGeachie JM, Davies MJ, Grounds MD . Evans blue dye as an in vivo marker of myofibre damage: optimizing parameters for detecting initial myofibre membrane permeability. J Anat 2002; 200: 69–79.
Liu F, Nishikawa M, Clemens PR, Huang L . Transfer of full-length Dmd to the diaphragm muscle of Dmdmdx/mdx mice through systemic administration of plasmid DNA. Mol Ther 2001; 4: 45–51.
Acknowledgements
We thank Professor Fucheng Sun for his technical supports in building the ultrasound-mediated hyperthermia system for the in vivo studies. This work was supported by the QiMingXing Young Investigator Award 00QA14014 to YHX.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Xu, L., Zhao, Y., Zhang, Q. et al. Regulation of transgene expression in muscles by ultrasound-mediated hyperthermia. Gene Ther 11, 894–900 (2004). https://doi.org/10.1038/sj.gt.3302254
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302254
Keywords
This article is cited by
-
Induced transgene expression for the treatment of solid tumors by hematopoietic stem cell-based gene therapy
Cancer Gene Therapy (2012)