Abstract
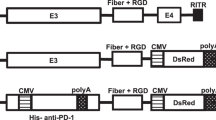
The use of recombinant adenovirus (Ad) vectors containing genetically modified capsid proteins is an attractive strategy for achieving targeted gene transfer. The HI loop of the fiber knob is a promising candidate location for the incorporation of foreign ligands for achieving this goal. However, the method of constructing an Ad vector containing a foreign ligand in the HI loop of the fiber knob has proved difficult. In this study, we developed a simple system to construct fiber-modified vectors. To do this, a vector plasmid containing a complete E1/E3-deleted Ad type 5 genome and a unique Csp45I and/or ClaI site between positions 32679 and 32680 of the Ad genome (residues threonine-546 and proline-547 of the fiber protein) was constructed. Oligonucleotides corresponding to the Arg-Gly-Asp (RGD) or Asn-Gly-Arg (NGR)-containing peptide motif (as a model) and containing a Csp45I and/or ClaI recognition site, were ligated into the Csp45I and/or ClaI-digested plasmid. The foreign transgene expression cassette was inserted into the E1 deletion site of the vector plasmid and the fiber-mutant Ad vector was produced by transfection of the PacI-digested plasmid into 293 cells. The virus containing the RGD or NGR peptide on the fiber knob was able to infect human glioma cells, which do not express coxsackievirus and adenovirus receptor (CAR), one of the Ad virus receptors, about 100–1000 times more efficient than the virus containing wild-type fiber. This suggested that the mutant virus mediated CAR-independent cell entry pathway. The simplicity of this method allows not only for easy construction of fiber-mutant Ad vectors, but also for screening of the peptides that target the vector to the desired cells and tissues.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kay MA, Woo SL . Gene therapy for metabolic disorders Trends Genet 1994 19: 253–257
Kozarsky KF, Wilson JM . Gene therapy: adenovirus vectors Curr Opin Genet Dev 1993 3: 499–503
Bergelson JM et al. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5 Science 1997 275: 1320–1323
Miller CR et al. Differential susceptibility of primary and established human glioma cells to adenovirus infection: targeting via the epidermal growth factor receptor achieves fiber receptor-independent gene transfer Cancer Res 1998 58: 5738–5748
Pickles RJ et al. Limited entry of adenovirus vectors into well-differentiated airway epithelium is responsible for inefficient gene transfer J Virol 1998 72: 6014–6023
Zabner J et al. Lack of high affinity fiber receptor activity explains the resistance of ciliated airway epithelia to adenovirus infection J Clin Invest 1997 100: 1144–1149
Henry LJ et al. Characterization of the knob domain of the adenovirus type 5 fiber protein expressed in Escherichia coli J Virol 1994 68: 5239–5246
Bai M, Harfe B, Freimuth P . Mutations that alter an Arg-Gly-Asp (RGD) sequence in the adenovirus type 2 penton baseprotein abolish its cell-rounding activity and delay virusreproduction in flat cells J Virol 1993 67: 5198–5205
Wickham TJ, Mathias P, Cheresh DA, Nemerow GR . Integrins avb3 and avb5 promote adenovirus internalization but not virus attachment Cell 1993 73: 309–319
Bouri K et al. Poly-lysine modification of adenoviral fiber protein enhances muscle cell transduction Hum Gene Ther 1999 10: 1633–1640
Gonzalez R et al. Increased gene transfer in acute myeloid leukemic cells by an adenovirus vector containing a modified fiber protein Gene Therapy 1999 6: 314–320
Wickham TJ et al. Increased in vitro and in vivo gene transfer by adenovirus vectors containing chimeric fiber proteins J Virol 1997 71: 8221–8229
Yoshida Y et al. Generation of fiber-mutant recombinant adenoviruses for gene therapy of malignant glioma Hum Gene Ther 1998 9: 2503–2515
Dmitriev I et al. An adenovirus vector with genetically modified fibers demonstrates expanded tropism via utilization of a coxsackievirus J Virol 1998 72: 9706–9713
Krasnykh V et al. Characterization of an adenovirus vector containing a heterologous peptide epitope in the HI loop of the fiber knob J Virol 1998 72: 1844–1852
Reynolds PN, Dmitriev I, Curiel DT . insertion of an RDG motif into the HI loop of adenovirus fiber protein alters the distribution of transgene expression of the systemically administered vector Gene Therapy 1999 6: 1336–1339
Hong JS, Engler JA . Domains required for assembly ofadenovirus type 2 fiber trimers J Virol 1996 70: 7071–7078
Xia D, Henry LJ, Gerard RD, Deisenhofer J . Crystal structure of the receptor-binding domain of adenovirus type 5 fiber protein at 1.7 A resolution Structure 1994 2: 1259–1270
Xia D, Henry L, Gerard RD, Deisenhofer J . Structure of the receptor binding domain of adenovirus type 5 fiber protein Curr Top Microbiol Immunol 1995 199: 39–46
Mizuguchi H, Kay MA . Efficient construction of a recombinant adenovirus vector by an improved in vitro ligation method Hum Gene Ther 1998 9: 2577–2583
Mizuguchi H, Kay MA . A simple method for constructing E1 and E1/E4 deleted recombinant adenovirus vector Hum Gene Ther 1999 10: 2013–2017
Arap W, Pasqualini R, Ruoslahti E . Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model Science 1998 279: 377–380
Pasqualini R, Koivunen E, Ruoslahti E . Alpha v integrins as receptors for tumor targeting by circulating ligands Nat Biotechnol 1997 15: 542–546
Koivunen E, Wang B, Ruoslahti E . Phage libraries displaying cyclic peptides with different ring sizes: ligand specificities of the RGD-directed integrins Biotechnology 1995 13: 265–270
Gimble FS, Thorner J . Homing of a DNA endonuclease gene by meiotic gene conversion in Saccharomyces cerevisiae Nature 1992 357: 301–306
Marshall P, Lemieux C . Cleavage pattern of the homing endonuclease encoded by the fifth intron in the chloroplast large subunit rRNA-encoding gene of Chlamydomonas eugametos Gene 1991 104: 241–245
Maizel JVJ, White DO, Scharff MD . The polypeptides of adenovirus. I. Evidence for multiple protein components in the virion and a comparison of types 2, 7A, and 12 Virology 1968 36: 115–125
Heffelfinger SC et al. SK HEP-1: a human cell line of endothelial origin In Vitro Cell Dev Biol 1992 28A: 136–142
Asaoka K et al. Dependence of efficient adenoviral gene delivery in malignant glioma cells on the expression levels of the Coxsackievirus and adenovirus receptor J Neurosurg 2000 92: 1002–1008
Pasqualini R et al. Aminopeptidase N is a receptor for tumor-homing peptides and a target for inhibiting angiogenesis Cancer Res 2000 60: 722–727
Barry MA, Dower WJ, Johnston SA . Toward cell-targeting gene therapy vectors: selection of cell-binding peptides from random peptide-presenting phage libraries Nat Med 1996 2: 299–305
Koivunen E et al. Tumor targeting with a selective gelatinase inhibitor Nat Biotechnol 1999 17: 768–774
Pasqualini R, Ruoslahti E . Organ targeting in vivo using phage display peptide libraries Nature 1996 380: 364–366
Douglas JT et al. A system for the propagation of adenoviral vectors with genetically modified receptor specificities Nat Biotechnol 1999 17: 470–475
Einfeld DA et al. Construction of a pseudoreceptor that mediates transduction by adenoviruses expressing a ligand in fiber or penton base J Virol 1999 73: 9130–9136
Roelvink PW et al. Identification of a conserved receptor-binding site on the fiber proteins of CAR-recognizing adenoviridae Science 1999 286: 1568–1571
Kirby I et al. Mutations in the DG loop of adenovirus type 5 fiber knob protein abolish high-affinity binding to its cellular receptor CAR J Virol 1999 73: 9508–9514
Acknowledgements
We would like to thank Jun Murai and Nobuko Heishi for technical assistance. We would like to thank Dr M Tada (Hokkaido University, Japan) and Dr JM Bergelson (The Children's Hospital of Philadelphia, PA) for kindly providing LN444 cells and mouse monoclonal antibody RmcB, respectively. This work was supported by grants from the Ministry of Health and Welfare in Japan and Grant-in-Aid for Scientific Research on Priority Areas (C). MAK was supported by NIH DK49022.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mizuguchi, H., Koizumi, N., Hosono, T. et al. A simplified system for constructing recombinant adenoviral vectors containing heterologous peptides in the HI loop of their fiber knob. Gene Ther 8, 730–735 (2001). https://doi.org/10.1038/sj.gt.3301453
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3301453
Keywords
This article is cited by
-
Effects of pre-existing anti-adenovirus antibodies on transgene expression levels and therapeutic efficacies of arming oncolytic adenovirus
Scientific Reports (2022)
-
Response of human melanoma cell lines to interferon-beta gene transfer mediated by a modified adenoviral vector
Scientific Reports (2020)
-
Improving Molecular Therapy in the Kidney
Molecular Diagnosis & Therapy (2020)
-
Antibodies against adenovirus fiber and penton base proteins inhibit adenovirus vector-mediated transduction in the liver following systemic administration
Scientific Reports (2018)
-
Reestablishment of p53/Arf and interferon-β pathways mediated by a novel adenoviral vector potentiates antiviral response and immunogenic cell death
Cell Death Discovery (2017)