Abstract
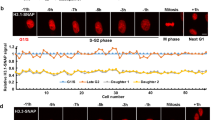
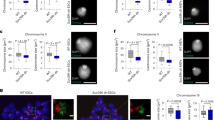
The histone variant H2A.Z plays an essential role in metazoans but its function remains to be determined. Here, we developed a new inducible RNAi strategy to elucidate the role of H2A.Z in mammalian cell lines. We show that in the absence of H2A.Z, the genome becomes highly unstable and that this instability is caused by defects in the chromosome segregation process. Analysis of H2A.Z localization reveals that in these cells it is enriched at heterochromatic foci with HP1α on the arms of chromosomes but not at centromeric regions. When H2A.Z is depleted, normal HP1α-chromatin interactions are disrupted on the chromosomal arms and, notably, also at pericentric regions. Therefore, H2A.Z controls the localization of HP1α. We conclude that H2A.Z is essential for the accurate transmission of chromosomes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Faast, R. et al. Histone variant H2A.Z is required for early mammalian development. Curr. Biol. 11, 1183–1187 (2001).
Santisteban, M.S., Kalashnikova, T. & Smith, M.M. Histone H2A.Z regulates transcription and is partially redundant with nucleosome remodeling complexes. Cell 103, 411–422 (2000).
Meneghini, M.D., Wu, M. & Madhani, H.D. Conserved histone variant H2A.Z protects euchromatin from the ectopic spread of silent heterochromatin. Cell 112, 725–736 (2003).
Madigan, J.P., Chotkowski, H.L. & Glaser, R.L. DNA double-strand break-induced phosphorylation of Drosophila histone variant H2Av helps prevent radiation-induced apoptosis. Nucleic Acids Res. 30, 3698–3705 (2002).
Rangasamy, D., Berven, L., Ridgway, P. & Tremethick, D.J. Pericentric heterochromatin becomes enriched with H2A.Z during early mammalian development. EMBO J. 22, 1599–1607 (2003).
Leach, T.J. et al. Histone H2A.Z is widely but nonrandomly distributed in chromosomes of Drosophila melanogaster. J. Biol. Chem. 275, 23267–23272 (2000).
No, D., Yao, T.P. & Evans, R.M. Ecdysone-inducible gene expression in mammalian cells and transgenic mice. Proc. Natl. Acad. Sci. USA 93, 3346–3351 (1996).
Zeng, Y., Wagner, E.J. & Cullen, B.R. Both natural and designed micro RNAs can inhibit the expression of cognate mRNAs when expressed in human cells. Mol. Cell 9, 1327–1333 (2002).
Elbashir, S.M. et al. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411, 494–498 (2001).
Ekwall, K., Cranston, G. & Allshire, R.C. Fission yeast mutants that alleviate transcriptional silencing in centromeric flanking repeats and disrupt chromosome segregation. Genetics 153, 1153–1169 (1999).
Maison, C. et al. Higher-order structure in pericentric heterochromatin involves a distinct pattern of histone modification and an RNA component. Nat. Genet. 30, 329–334 (2002).
Cimini, D., Mattiuzzo, M., Torosantucci, L. & Degrassi, F. Histone hyperacetylation in mitosis prevents sister chromatid separation and produces chromosome segregation defects. Mol. Biol. Cell 14, 3821–3833 (2003).
Ekwall, K. et al. The chromodomain protein Swi6: a key component at fission yeast centromeres. Science 269, 1429–1431 (1995).
Carr, A.M. et al. Analysis of a histone H2A variant from fission yeast: evidence for a role in chromosome stability. Mol. Gen. Genet. 245, 628–635 (1994).
Nonaka, N. et al. Recruitment of cohesin to heterochromatic regions by Swi6/HP1 in fission yeast. Nat. Cell Biol. 4, 89–93 (2002).
Nasmyth, K. Segregating sister genomes: the molecular biology of chromosome separation. Science 297, 559–565 (2002).
Bernard, P. & Allshire, R. Centromeres become unstuck without heterochromatin. Trends Cell Biol. 12, 419–424 (2002).
Antonio, C. et al. Xkid, a chromokinesin required for chromosome alignment on the metaphase plate. Cell 102, 425–435 (2000).
Levesque, A.A. & Compton, D.A. The chromokinesin Kid is necessary for chromosome arm orientation and oscillation, but not congression, on mitotic spindles. J. Cell Biol. 154, 1135–1146 (2001).
Adams, R.R., Carmena, M. & Earnshaw, W.C. Chromosomal passengers and the (aurora) ABCs of mitosis. Trends Cell Biol. 11, 49–54 (2001).
Pinto, I. & Winston, F. Histone H2A is required for normal centromere function in Saccharomyces cerevisiae. EMBO J. 19, 1598–1612 (2000).
de la Barre, A.E., Angelov, D., Molla, A. & Dimitrov, S. The N-terminus of histone H2B, but not that of histone H3 or its phosphorylation, is essential for chromosome condensation. EMBO J. 20, 6383–6393 (2001).
Fan, J.Y., Gordon, F., Luger, K., Hansen, J.C. & Tremethick, D.J. The essential histone variant H2A.Z regulates the equilibrium between different chromatin conformational states. Nat. Struct. Biol. 9, 172–176 (2002).
Miyagishi, M. & Taira, K. U6 promoter-driven siRNAs with four uridine 3′ overhangs efficiently suppress targeted gene expression in mammalian cells. Nat. Biotechnol. 20, 497–500 (2002).
Minc, E., Allory, Y., Courvalin, J.C. & Buendia, B. Immunolocalization of HP1 proteins in metaphasic mammalian chromosomes. Methods Cell Sci. 23, 171–174 (2001).
Gruss, O.J. et al. Chromosome-induced microtubule assembly mediated by TPX2 is required for spindle formation in HeLa cells. Nat. Cell Biol. 4, 871–879 (2002).
Gupta, S., Schoer, R.A., Egan, J.E., Hannon, G.J. & Mittal, V. Inducible, reversible, and stable RNA interference in mammalian cells. Proc. Natl. Acad. Sci. USA 101, 1927–1932, (2004).
Acknowledgements
This work was supported by a grant from the Australian National Health and Medical Research Council to D.T. We thank P. Ridgway for critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
H2A.Z expression can be inhibited by siRNA. (PDF 207 kb)
Supplementary Fig. 2
Inducible synthesis of H2A.Z siRNA. (PDF 151 kb)
Rights and permissions
About this article
Cite this article
Rangasamy, D., Greaves, I. & Tremethick, D. RNA interference demonstrates a novel role for H2A.Z in chromosome segregation. Nat Struct Mol Biol 11, 650–655 (2004). https://doi.org/10.1038/nsmb786
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb786
This article is cited by
-
Knockout tales: the versatile roles of histone H3.3 in development and disease
Epigenetics & Chromatin (2023)
-
Histone variant H2A.Z modulates nucleosome dynamics to promote DNA accessibility
Nature Communications (2023)
-
Loss of TIP60 (KAT5) abolishes H2AZ lysine 7 acetylation and causes p53, INK4A, and ARF-independent cell cycle arrest
Cell Death & Disease (2022)
-
H2A.Z acetylation by lincZNF337-AS1 via KAT5 implicated in the transcriptional misregulation in cancer signaling pathway in hepatocellular carcinoma
Cell Death & Disease (2021)
-
Multiple roles of H2A.Z in regulating promoter chromatin architecture in human cells
Nature Communications (2021)