Key Points
-
In vivo imaging allows for the observation of the behaviour of single cells in the diseased nervous system. This can provide insights into how neurological diseases emerge and how they can be treated.
-
In vivo microscopy with single-cell resolution is based on progress in two fields. First, multiphoton microscopy, which provides high resolution views from deep within the nervous system. Second, transgenic mice with fluorescent neurons and glial cells, which allow for long-term observations with low toxicity. Combining these techniques now make in vivo observations possible in many neurological disease models.
-
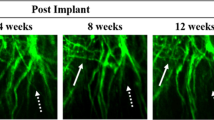
In vivo imaging in models of neurotrauma has been used to study axonal degeneration and regeneration. One insight from these studies is the importance of glial guidance for successful regeneration in the PNS. By contrast, in the CNS, growing axons appear to lack such guidance, and degeneration outweighs axonal regrowth.
-
In the area of neurodegenerative diseases, in vivo microscopy studies have mainly focused on Alzheimer's disease models. Fluorescent dyes, which bind to amyloid-β, allow the analysis of the kinetics of plaque formation and clearance, as well as the evolution of plaque-related neuronal damage.
-
Multiphoton imaging is a convenient tool with which to study the brain's microvasculature by enabling the measurement of local flow and providing the means to specifically occlude single microvessels. This method has revealed the degree of haemodynamic compensation that occurs after vessel occlusion. Furthermore, co-visualization of blood flow and neuronal morphology can help to define how neurons are damaged by varying degrees of ischaemia.
-
The transmigration of immune cells across the blood–brain barrier is a crucial step in the initiation of neuroinflammatory diseases such as multiple sclerosis. By labelling immune cells ex vivo and co-visualizing the nervous system vasculature, transmigration can be readily studied with in vivo imaging. Imaging of immune cell invasion has helped to define the role of specific adhesion molecules in transmigration and has guided the development of therapies that restrict immune cell influx.
-
Functional microscopy techniques, such as intrinsic optical imaging and calcium imaging, allow the measurment of neuronal activity in vivo. Using these approaches, epileptogenic foci can be mapped, and aberrant patterns of activity can be recorded in single cells.
-
Important challenges remain for the future development of optical in vivo imaging in neurological diseases. One challenge is to address the molecular mechanisms that cause disease and provide targets for therapy. Another is to relate insights derived from imaging animal models at cellular resolution with the clinical presentation of human patients.
Abstract
In vivo microscopy is an exciting tool for neurological research because it can reveal how single cells respond to damage of the nervous system. This helps us to understand how diseases unfold and how therapies work. Here, we review the optical imaging techniques used to visualize the different parts of the nervous system, and how they have provided fresh insights into the aetiology and therapeutics of neurological diseases. We focus our discussion on five areas of neuropathology (trauma, degeneration, ischaemia, inflammation and seizures) in which in vivo microscopy has had the greatest impact. We discuss the challenging issues in the field, and argue that the convergence of new optical and non-optical methods will be necessary to overcome these challenges.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Lichtman, J. W. & Fraser, S. E. The neuronal naturalist: watching neurons in their native habitat. Nature Neurosci. 4 (Suppl.), 1215–1220 (2001). An overview of the conceptual advantages of in vivo imaging studies in the nervous system.
Trachtenberg, J. T. et al. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature 420, 788–794 (2002).
Grutzendler, J., Kasthuri, N. & Gan, W. B. Long-term dendritic spine stability in the adult cortex. Nature 420, 812–816 (2002).
Davalos, D. et al. ATP mediates rapid microglial response to local brain injury in vivo. Nature Neurosci. 8, 752–758 (2005).
Nimmerjahn, A., Kirchhoff, F. & Helmchen, F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308, 1314–1318 (2005). References 4 and 5 are the first reports to illustrate the remarkable in vivo dynamics of microglia cells in the brain.
Steward, O., Zheng, B. & Tessier-Lavigne, M. False resurrections: distinguishing regenerated from spared axons in the injured central nervous system. J. Comp. Neurol. 459, 1–8 (2003).
Medana, I., Martinic, M. A., Wekerle, H. & Neumann, H. Transection of major histocompatibility complex class I-induced neurites by cytotoxic T lymphocytes. Am. J. Pathol. 159, 809–815 (2001).
Merrill, J. E. & Scolding, N. J. Mechanisms of damage to myelin and oligodendrocytes and their relevance to disease. Neuropathol. Appl. Neurobiol. 25, 435–458 (1999).
Griffiths, I. et al. Axonal swellings and degeneration in mice lacking the major proteolipid of myelin. Science 280, 1610–1613 (1998).
Bjartmar, C., Wujek, J. R. & Trapp, B. D. Axonal loss in the pathology of MS: consequences for understanding the progressive phase of the disease. J. Neurol. Sci. 206, 165–171 (2003).
Jensen, M. B., Hegelund, I. V., Lomholt, N. D., Finsen, B. & Owens, T. IFNγ enhances microglial reactions to hippocampal axonal degeneration. J. Neurosci. 20, 3612–3621 (2000).
Bacskai, B. J. et al. Imaging of amyloid-ß deposits in brains of living mice permits direct observation of clearance of plaques with immunotherapy. Nature Med. 7, 369–372 (2001). Introduces important tools for in vivo multiphoton studies in animal models of Alzheimer's disease.
Weiss, P. A. Neuronal dynamics and axonal flow: axonal peristalsis. Proc. Natl Acad. Sci. USA 69, 1309–1312 (1972).
Bray, D. Surface movements during the growth of single explanted neurons. Proc. Natl Acad. Sci. USA 65, 905–910 (1970).
Lichtman, J. W. & Conchello, J. A. Fluorescence microscopy. Nature Methods 2, 910–919 (2005).
Denk, W., Strickler, J. H. & Webb, W. W. Two-photon laser scanning fluorescence microscopy. Science 248, 73–76 (1990).
Zipfel, W. R., Williams, R. M. & Webb, W. W. Nonlinear magic: multiphoton microscopy in the biosciences. Nature Biotechnol. 21, 1369–1377 (2003).
Helmchen, F. & Denk, W. Deep tissue two-photon microscopy. Nature Methods 2, 932–940 (2005).
Theer, P., Hasan, M. T. & Denk, W. Two-photon imaging to a depth of 1000 μm in living brains by use of a Ti:Al2O3 regenerative amplifier. Opt. Lett. 28, 1022–1024 (2003).
Mizrahi, A., Crowley, J. C., Shtoyerman, E. & Katz, L. C. High-resolution in vivo imaging of hippocampal dendrites and spines. J. Neurosci. 24, 3147–3151 (2004).
Levene, M. J., Dombeck, D. A., Kasischke, K. A., Molloy, R. P. & Webb, W. W. In vivo multiphoton microscopy of deep brain tissue. J. Neurophysiol. 91, 1908–1912 (2004).
Helmchen, F., Fee, M. S., Tank, D. W. & Denk, W. A miniature head-mounted two-photon microscope. High-resolution brain imaging in freely moving animals. Neuron 31, 903–912 (2001). Describes a new approach for in vivo imaging with cellular resolution in awake, behaving rodents.
Flusberg, B. A. et al. Fiber-optic fluorescence imaging. Nature Methods 2, 941–950 (2005).
Young, P. & Feng, G. Labeling neurons in vivo for morphological and functional studies. Curr. Opin. Neurobiol. 14, 642–646 (2004).
Feng, G. et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron 28, 41–51 (2000). The introduction of Thy1-XFP transgenic mice, which are the basis for most in vivo imaging studies of neurons.
Portera-Cailliau, C., Weimer, R. M., De Paola, V., Caroni, P. & Svoboda, K. Diverse modes of axon elaboration in the developing neocortex. PLoS Biol. 3, e272 (2005).
Lee, W. C. et al. Dynamic remodeling of dendritic arbors in GABAergic interneurons of adult visual cortex. PLoS Biol. 4, e29 (2005).
Keller-Peck, C. R. et al. Asynchronous synapse elimination in neonatal motor units: studies using GFP transgenic mice. Neuron 31, 381–394 (2001).
Bareyre, F. M., Kerschensteiner, M., Misgeld, T. & Sanes, J. R. Transgenic labeling of the corticospinal tract for monitoring axonal responses to spinal cord injury. Nature Med. 11, 1355–1360 (2005).
Vives, V., Alonso, G., Solal, A. C., Joubert, D. & Legraverend, C. Visualization of S100B-positive neurons and glia in the central nervous system of EGFP transgenic mice. J. Comp. Neurol. 457, 404–419 (2003).
Zuo, Y. et al. Fluorescent proteins expressed in mouse transgenic lines mark subsets of glia, neurons, macrophages, and dendritic cells for vital examination. J. Neurosci. 24, 10999–11009 (2004).
Zhuo, L. et al. Live astrocytes visualized by green fluorescent protein in transgenic mice. Dev. Biol. 187, 36–42 (1997).
Nolte, C. et al. GFAP promoter-controlled EGFP-expressing transgenic mice: a tool to visualize astrocytes and astrogliosis in living brain tissue. Glia 33, 72–86 (2001).
Hirrlinger, P. G. et al. Expression of reef coral fluorescent proteins in the central nervous system of transgenic mice. Mol. Cell. Neurosci. 30, 291–303 (2005).
Fuss, B. et al. Purification and analysis of in vivo-differentiated oligodendrocytes expressing the green fluorescent protein. Dev. Biol. 218, 259–274 (2000).
Jung, S. et al. Analysis of fractalkine receptor CX3CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol. Cell. Biol. 20, 4106–4114 (2000).
Mempel, T. R., Scimone, M. L., Mora, J. R. & von Andrian, U. H. In vivo imaging of leukocyte trafficking in blood vessels and tissues. Curr. Opin. Immunol. 16, 406–417 (2004).
Dittgen, T. et al. Lentivirus-based genetic manipulations of cortical neurons and their optical and electrophysiological monitoring in vivo. Proc. Natl Acad. Sci. USA 101, 18206–18211 (2004).
Flugel, A., Willem, M., Berkowicz, T. & Wekerle, H. Gene transfer into CD4+ T lymphocytes: green fluorescent protein-engineered, encephalitogenic T cells illuminate brain autoimmune responses. Nature Med. 5, 843–847 (1999).
Borrell, V., Yoshimura, Y. & Callaway, E. M. Targeted gene delivery to telencephalic inhibitory neurons by directional in utero electroporation. J. Neurosci. Methods 143, 151–158 (2005).
Haas, K., Sin, W. C., Javaherian, A., Li, Z. & Cline, H. T. Single-cell electroporation for gene transfer in vivo. Neuron 29, 583–591 (2001).
Nimmerjahn, A., Kirchhoff, F., Kerr, J. N. & Helmchen, F. Sulforhodamine 101 as a specific marker of astroglia in the neocortex in vivo. Nature Methods 1, 31–37 (2004).
LaMantia, A. S. & Purves, D. Development of glomerular pattern visualized in the olfactory bulbs of living mice. Nature 341, 646–649 (1989).
Williams, P. L. & Hall, S. M. In vivo observations on mature myelinated nerve fibres of the mouse. J. Anat. 107, 31–38 (1970).
Maiti, S., Shear, J. B., Williams, R. M., Zipfel, W. R. & Webb, W. W. Measuring serotonin distribution in live cells with three-photon excitation. Science 275, 530–532 (1997).
Magrassi, L., Purves, D. & Lichtman, J. W. Fluorescent probes that stain living nerve terminals. J. Neurosci. 7, 1207–1214 (1987).
Lichtman, J. W., Wilkinson, R. S. & Rich, M. M. Multiple innervation of tonic endplates revealed by activity-dependent uptake of fluorescent probes. Nature 314, 357–359 (1985).
Cochilla, A. J., Angleson, J. K. & Betz, W. J. Monitoring secretory membrane with FM1–43 fluorescence. Annu. Rev. Neurosci. 22, 1–10 (1999).
De, G. F. et al. Targeting GFP to organelles. Methods Cell Biol. 58, 75–85 (1999).
DePaola, V., Arber, S. & Caroni, P. AMPA receptors regulate dynamic equilibrium of presynaptic terminals in mature hippocampal networks. Nature Neurosci. 6, 491–500 (2003).
Umemori, H., Linhoff, M. W., Ornitz, D. M. & Sanes, J. R. FGF22 and its close relatives are presynaptic organizing molecules in the mammalian brain. Cell 118, 257–270 (2004).
Li, Z. et al. Synaptic vesicle recycling studied in transgenic mice expressing synaptopHluorin. Proc. Natl Acad. Sci. USA 102, 6131–6136 (2005).
Miesenbock, G. Genetic methods for illuminating the function of neural circuits. Curr. Opin. Neurobiol. 14, 395–402 (2004).
Bonhoeffer, T. & Grinvald, A. The layout of iso-orientation domains in area 18 of cat visual cortex: optical imaging reveals a pinwheel-like organization. J. Neurosci. 13, 4157–4180 (1993).
Sasaki, S. et al. Optical imaging of intrinsic signals induced by peripheral nerve stimulation in the in vivo rat spinal cord. Neuroimage 17, 1240–1255 (2002).
Haglund, M. M., Ojemann, G. A. & Hochman, D. W. Optical imaging of epileptiform and functional activity in human cerebral cortex. Nature 358, 668–671 (1992). This study was one of the first to show that intrinsic optical imaging can be used to generate maps of epileptiform or physiological activity in the human cortex.
Konnerth, A. & Yuste, R. Imaging in Neurosciences and Development — a Laboratory Manual (Cold Spring Harbor Laboratory Press, New York, 2006).
Svoboda, K., Denk, W., Kleinfeld, D. & Tank, D. W. In vivo dendritic calcium dynamics in neocortical pyramidal neurons. Nature 385, 161–165 (1997). Illustrates that multiphoton imaging can be used to follow stimulation-induced calcium dynamics in cortical dendrites in vivo.
Yuste, R. & Katz, L. C. Control of postsynaptic Ca2+ influx in developing neocortex by excitatory and inhibitory neurotransmitters. Neuron 6, 333–344 (1991).
Stosiek, C., Garaschuk, O., Holthoff, K. & Konnerth, A. In vivo two-photon calcium imaging of neuronal networks. Proc. Natl Acad. Sci. USA 100, 7319–7324 (2003). Introduces the 'multicell bolus loading' technique that is used widely to label neurons and glial cells with calcium indicators in vivo.
Ohki, K., Chung, S., Ch'ng, Y. H., Kara, P. & Reid, R. C. Functional imaging with cellular resolution reveals precise micro-architecture in visual cortex. Nature 433, 597–603 (2005).
Adelsberger, H., Garaschuk, O. & Konnerth, A. Cortical calcium waves in resting newborn mice. Nature Neurosci. 8, 988–990 (2005).
Hasan, M. T. et al. Functional fluorescent Ca2+ indicator proteins in transgenic mice under TET control. PLoS Biol. 2, e163 (2004).
Miyawaki, A. Innovations in the imaging of brain functions using fluorescent proteins. Neuron 48, 189–199 (2005).
Dombeck, D. A., Blanchard-Desce, M. & Webb, W. W. Optical recording of action potentials with second-harmonic generation microscopy. J. Neurosci. 24, 999–1003 (2004).
Nuriya, M., Jiang, J., Nemet, B., Eisenthal, K. B. & Yuste, R. Imaging membrane potential in dendritic spines. Proc. Natl Acad. Sci. USA 103, 786–790 (2006).
Kuhn, B., Fromherz, P. & Denk, W. High sensitivity of Stark-shift voltage-sensing dyes by one- or two-photon excitation near the red spectral edge. Biophys. J. 87, 631–639 (2004).
Guerrero, G., Siegel, M. S., Roska, B., Loots, E. & Isacoff, E. Y. Tuning FlaSh: redesign of the dynamics, voltage range, and color of the genetically encoded optical sensor of membrane potential. Biophys. J. 83, 3607–3618 (2002).
Hollander, H. & Mehraein, P. On the mechanics of myelin sphere formation in Wallerian degeneration. Intravital microscopic studies of single degenerating motor fibers of the frog. Z. Zellforsch. Mikrosk. Anat. 72, 276–280 (1966).
Williams, P. L. & Hall, S. M. Prolonged in vivo observations of normal peripheral nerve fibres and their acute reactions to crush and deliberate trauma. J. Anat. 108, 397–408 (1971).
Coleman, M. Axon degeneration mechanisms: commonality amid diversity. Nature Rev. Neurosci. 6, 889–898 (2005).
Nguyen, Q. T., Sanes, J. R. & Lichtman, J. W. Pre-existing pathways promote precise projection patterns. Nature Neurosci. 5, 861–867 (2002).
Pan, Y. A., Misgeld, T., Lichtman, J. W. & Sanes, J. R. Effects of neurotoxic and neuroprotective agents on peripheral nerve regeneration assayed by time-lapse imaging in vivo. J. Neurosci. 23, 11479–11488 (2003).
Kerschensteiner, M., Schwab, M. E., Lichtman, J. W. & Misgeld, T. In vivo imaging of axonal degeneration and regeneration in the injured spinal cord. Nature Med. 11, 572–577 (2005).
Wang, X. et al. P2X7 receptor inhibition improves recovery after spinal cord injury. Nature Med. 10, 821–827 (2004).
Bhatt, D. H., Otto, S. J., Depoister, B. & Fetcho, J. R. Cyclic AMP-induced repair of zebrafish spinal circuits. Science 305, 254–258 (2004). Shows that in vivo imaging can help to document axonal regeneration and functional reconnection in the CNS of zebrafish.
Qiu, J. et al. Spinal axon regeneration induced by elevation of cyclic AMP. Neuron 34, 895–903 (2002).
Galbraith, J. A. & Terasaki, M. Controlled damage in thick specimens by multiphoton excitation. Mol. Biol. Cell 14, 1808–1817 (2003).
Yanik, M. F. et al. Neurosurgery: functional regeneration after laser axotomy. Nature 432, 822 (2004).
Christie, R. H. et al. Growth arrest of individual senile plaques in a model of Alzheimer's disease observed by in vivo multiphoton microscopy. J. Neurosci. 21, 858–864 (2001).
McLellan, M. E., Kajdasz, S. T., Hyman, B. T. & Bacskai, B. J. In vivo imaging of reactive oxygen species specifically associated with thioflavine S-positive amyloid plaques by multiphoton microscopy. J. Neurosci. 23, 2212–2217 (2003).
Tsai, J., Grutzendler, J., Duff, K. & Gan, W. B. Fibrillar amyloid deposition leads to local synaptic abnormalities and breakage of neuronal branches. Nature Neurosci. 7, 1181–1183 (2004).
Spires, T. L. et al. Dendritic spine abnormalities in amyloid precursor protein transgenic mice demonstrated by gene transfer and intravital multiphoton microscopy. J. Neurosci. 25, 7278–7287 (2005).
Brendza, R. P. et al. Anti-Aβ antibody treatment promotes the rapid recovery of amyloid-associated neuritic dystrophy in PDAPP transgenic mice. J. Clin. Invest. 115, 428–433 (2005).
Stern, E. A. et al. Cortical synaptic integration in vivo is disrupted by amyloid-β; plaques. J. Neurosci. 24, 4535–4540 (2004).
Schaefer, A. M., Sanes, J. R. & Lichtman, J. W. A compensatory subpopulation of motor neurons in a mouse model of amyotrophic lateral sclerosis. J. Comp. Neurol. 490, 209–219 (2005).
Holzbaur, E. L. Motor neurons rely on motor proteins. Trends Cell Biol. 14, 233–240 (2004).
Yin, X. et al. Dysmyelinated lower motor neurons retract and regenerate dysfunctional synaptic terminals. J. Neurosci. 24, 3890–3898 (2004).
Villringer, A., Them, A., Lindauer, U., Einhaupl, K. & Dirnagl, U. Capillary perfusion of the rat brain cortex. An in vivo confocal microscopy study. Circ. Res. 75, 55–62 (1994).
Hudetz, A. G. Blood flow in the cerebral capillary network: a review emphasizing observations with intravital microscopy. Microcirculation 4, 233–252 (1997).
Kleinfeld, D., Mitra, P. P., Helmchen, F. & Denk, W. Fluctuations and stimulus-induced changes in blood flow observed in individual capillaries in layers 2 through 4 of rat neocortex. Proc. Natl Acad. Sci. USA 95, 15741–15746 (1998).
Schaffer, C. B. et al. Two-photon imaging of cortical surface microvessels reveals a robust redistribution in blood flow after vascular occlusion. PLoS Biol. 4, e22 (2006).
Nishimura, N. et al. Targeted insult to subsurface cortical blood vessels using ultrashort laser pulses: three models of stroke. Nature Methods 3, 99–108 (2006). Together with reference 92, this publication introduces a new multiphoton-based toolkit to study vessel occlusion and ischaemia in the brain.
Zhang, Z. G. et al. A model of mini-embolic stroke offers measurements of the neurovascular unit response in the living mouse. Stroke 36, 2701–2704 (2005).
Zhang, S., Boyd, J., Delaney, K. & Murphy, T. H. Rapid reversible changes in dendritic spine structure in vivo gated by the degree of ischemia. J. Neurosci. 25, 5333–5338 (2005).
Iadecola, C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nature Rev. Neurosci. 5, 347–360 (2004).
Takano, T. et al. Astrocyte-mediated control of cerebral blood flow. Nature Neurosci. 9, 260–267 (2006).
Zonta, M. et al. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nature Neurosci. 6, 43–50 (2003).
Mulligan, S. J. & MacVicar, B. A. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature 431, 195–199 (2004).
Vajkoczy, P., Laschinger, M. & Engelhardt, B. α4-integrin-VCAM-1 binding mediates G protein-independent capture of encephalitogenic T cell blasts to CNS white matter microvessels. J. Clin. Invest. 108, 557–565 (2001). Introduction of a spinal cord window technique that provides a direct view of the spinal microcirculation, and that is used to study the molecular interactions underlying transmigration of encephalitogenic T cells.
Piccio, L. et al. Molecular mechanisms involved in lymphocyte recruitment in inflamed brain microvessels: critical roles for P-selectin glycoprotein ligand-1 and heterotrimeric Gi-linked receptors. J. Immunol. 168, 1940–1949 (2002).
dos Santos, A. C. et al. CCL2 and CCL5 mediate leukocyte adhesion in experimental autoimmune encephalomyelitis – an intravital microscopy study. J. Neuroimmunol. 162, 122–129 (2005).
Miller, D. H. et al. A controlled trial of natalizumab for relapsing multiple sclerosis. N. Engl. J. Med. 348, 15–23 (2003).
Lapointe, B. M., Herx, L. M., Gill, V., Metz, L. M. & Kubes, P. IVIg therapy in brain inflammation: etiology-dependent differential effects on leucocyte recruitment. Brain 127, 2649–2656 (2004).
Nitsch, R. et al. Direct impact of T cells on neurons revealed by two-photon microscopy in living brain tissue. J. Neurosci. 24, 2458–2464 (2004).
Kawakami, N. et al. Live imaging of effector cell trafficking and autoantigen recognition within the unfolding autoimmune encephalomyelitis lesion. J. Exp. Med. 201, 1805–1814 (2005).
Rich, M. M., Colman, H. & Lichtman, J. W. In vivo imaging shows loss of synaptic sites from neuromuscular junctions in a model of myasthenia gravis. Neurology 44, 2138–2145 (1994).
Schwartz, T. H. & Bonhoeffer, T. In vivo optical mapping of epileptic foci and surround inhibition in ferret cerebral cortex. Nature Med. 7, 1063–1067 (2001).
Badea, T., Goldberg, J., Mao, B. & Yuste, R. Calcium imaging of epileptiform events with single-cell resolution. J. Neurobiol. 48, 215–227 (2001).
Kerr, J. N., Greenberg, D. & Helmchen, F. Imaging input and output of neocortical networks in vivo. Proc. Natl Acad. Sci. USA 102, 14063–14068 (2005).
Tian, G. F. et al. An astrocytic basis of epilepsy. Nature Med. 11, 973–981 (2005). Uses in vivo calcium imaging to reveal a potential role of astrocytic calcium signalling in epileptiform neuronal activity, and shows that antiepileptic drugs influence astrocytic calcium spikes.
Rensing, N. et al. In vivo imaging of dendritic spines during electrographic seizures. Ann. Neurol. 58, 888–898 (2005).
Hirase, H., Creso, J. & Buzsaki, G. Capillary level imaging of local cerebral blood flow in bicuculline-induced epileptic foci. Neuroscience 128, 209–216 (2004).
Sullivan, M. R., Nimmerjahn, A., Sarkisov, D. V., Helmchen, F. & Wang, S. S. In vivo calcium imaging of circuit activity in cerebellar cortex. J. Neurophysiol. 94, 1636–1644 (2005).
Nimchinsky, E. A., Oberlander, A. M. & Svoboda, K. Abnormal development of dendritic spines in FMR1 knock-out mice. J. Neurosci. 21, 5139–5146 (2001).
Seehafer, S. S. & Pearce, D. A. You say lipofuscin, we say ceroid: defining autofluorescent storage material. Neurobiol. Aging 27, 576–588 (2006).
Ouardouz, M. et al. Depolarization-induced Ca2+ release in ischemic spinal cord white matter involves L-type Ca2+ channel activation of ryanodine receptors. Neuron 40, 53–63 (2003).
Micu, I. et al. NMDA receptors mediate calcium accumulation in myelin during chemical ischaemia. Nature 439, 988–992 (2006).
Laxman, B. et al. Noninvasive real-time imaging of apoptosis. Proc. Natl Acad. Sci. USA 99, 16551–16555 (2002).
Kadurugamuwa, J. L. et al. Reduction of astrogliosis by early treatment of pneumococcal meningitis measured by simultaneous imaging, in vivo, of the pathogen and host response. Infect. Immun. 73, 7836–7843 (2005).
Lin, A. H. et al. Global analysis of Smad2/3-dependent TGF-β signaling in living mice reveals prominent tissue-specific responses to injury. J. Immunol. 175, 547–554 (2005).
Bremer, C., Tung, C. H. & Weissleder, R. In vivo molecular target assessment of matrix metalloproteinase inhibition. Nature Med. 7, 743–748 (2001).
Lindsten, K., Menendez-Benito, V., Masucci, M. G. & Dantuma, N. P. A transgenic mouse model of the ubiquitin/proteasome system. Nature Biotechnol. 21, 897–902 (2003).
Ntziachristos, V., Ripoll, J., Wang, L. V. & Weissleder, R. Looking and listening to light: the evolution of whole-body photonic imaging. Nature Biotechnol. 23, 313–320 (2005). A comprehensive introduction to biophotonic imaging techniques and their current capabilities and limitations.
Michalet, X. et al. Quantum dots for live cells, in vivo imaging, and diagnostics. Science 307, 538–544 (2005).
So, M. K., Xu, C., Loening, A. M., Gambhir, S. S. & Rao, J. Self-illuminating quantum dot conjugates for in vivo imaging. Nature Biotechnol. 24, 339–343 (2006).
Zipfel, W. R. et al. Live tissue intrinsic emission microscopy using multiphoton-excited native fluorescence and second harmonic generation. Proc. Natl Acad. Sci. USA 100, 7075–7080 (2003).
Kasischke, K. A., Vishwasrao, H. D., Fisher, P. J., Zipfel, W. R. & Webb, W. W. Neural activity triggers neuronal oxidative metabolism followed by astrocytic glycolysis. Science 305, 99–103 (2004).
Campagnola, P. J. & Loew, L. M. Second-harmonic imaging microscopy for visualizing biomolecular arrays in cells, tissues and organisms. Nature Biotechnol. 21, 1356–1360 (2003).
Wallrabe, H. & Periasamy, A. Imaging protein molecules using FRET and FLIM microscopy. Curr. Opin. Biotechnol. 16, 19–27 (2005).
Stockholm, D. et al. Imaging calpain protease activity by multiphoton FRET in living mice. J. Mol. Biol. 346, 215–222 (2005). An example of multiphoton FRET imaging in living mice to visualize the kinetics of calpain activation in muscle on the basis of a genetic sensor.
Yasuda, R. et al. Supersensitive Ras activation in dendrites and spines revealed by two-photon fluorescence lifetime imaging. Nature Neurosci. 9, 283–291 (2006).
Masters, B. R. & Bohnke, M. Three-dimensional confocal microscopy of the living human eye. Annu. Rev. Biomed. Eng. 4, 69–91 (2002).
Cordeiro, M. F. et al. Real-time imaging of single nerve cell apoptosis in retinal neurodegeneration. Proc. Natl Acad. Sci. USA 101, 13352–13356 (2004).
Paques, M. et al. High resolution fundus imaging by confocal scanning laser ophthalmoscopy in the mouse. Vision Res. 46, 1336–1345 (2006).
Imanishi, Y., Batten, M. L., Piston, D. W., Baehr, W. & Palczewski, K. Noninvasive two-photon imaging reveals retinyl ester storage structures in the eye. J. Cell Biol. 164, 373–383 (2004).
Drexler, W. et al. Ultrahigh-resolution ophthalmic optical coherence tomography. Nature Med. 7, 502–507 (2001).
Zhang, J. et al. Mapping postnatal mouse brain development with diffusion tensor microimaging. Neuroimage 26, 1042–1051 (2005).
Sundgren, P. C. et al. Diffusion tensor imaging of the brain: review of clinical applications. Neuroradiology 46, 339–350 (2004).
Pautler, R. G., Mongeau, R. & Jacobs, R. E. In vivo trans-synaptic tract tracing from the murine striatum and amygdala utilizing manganese enhanced MRI (MEMRI). Magn. Reson. Med. 50, 33–39 (2003).
Caramanos, Z., Narayanan, S. & Arnold, D. L. 1H-MRS quantification of tNA and tCr in patients with multiple sclerosis: a meta-analytic review. Brain 128, 2483–2506 (2005).
Natt, O. et al. High-resolution 3D MRI of mouse brain reveals small cerebral structures in vivo. J. Neurosci. Methods 120, 203–209 (2002).
Wang, Y., Zhang, J., Mori, S. & Nathans, J. Axonal growth and guidance defects in Frizzled3 knock-out mice: a comparison of diffusion tensor magnetic resonance imaging, neurofilament staining, and genetically directed cell labeling. J. Neurosci. 26, 355–364 (2006). An interesting comparison of non-optical and optical techniques that illustrates the recent advances of diffusion tensor imaging of axonal tracts and shows how such non-optical approaches could converge with light microscopic techniques.
Bilgen, M. et al. Electrical stimulation of cortex improves corticospinal tract tracing in rat spinal cord using manganese-enhanced MRI. J. Neurosci. Methods 9 Mar 2006 (doi:10.1016/j.jneumeth.2006.02.001).
Bendszus, M. et al. Assessment of nerve degeneration by gadofluorine M-enhanced magnetic resonance imaging. Ann. Neurol. 57, 388–395 (2005).
Jack, C. R. Jr et al. In vivo magnetic resonance microimaging of individual amyloid plaques in Alzheimer's transgenic mice. J. Neurosci. 25, 10041–10048 (2005).
Pautler, R. G. & Fraser, S. E. The year(s) of the contrast agent — micro-MRI in the new millennium. Curr. Opin. Immunol. 15, 385–392 (2003).
Jasanoff, A. Functional MRI using molecular imaging agents. Trends Neurosci. 28, 120–126 (2005).
Bendszus, M. & Stoll, G. Caught in the act: in vivo mapping of macrophage infiltration in nerve injury by magnetic resonance imaging. J. Neurosci. 23, 10892–10896 (2003).
Anderson, S. A. et al. Magnetic resonance imaging of labeled T-cells in a mouse model of multiple sclerosis. Ann. Neurol. 55, 654–659 (2004).
Modo, M. et al. Tracking transplanted stem cell migration using bifunctional, contrast agent-enhanced, magnetic resonance imaging. Neuroimage 17, 803–811 (2002).
Zhao, M., Beauregard, D. A., Loizou, L., Davletov, B. & Brindle, K. M. Non-invasive detection of apoptosis using magnetic resonance imaging and a targeted contrast agent. Nature Med. 7, 1241–1244 (2001).
Klunk, W. E. et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann. Neurol. 55, 306–319 (2004).
Tsien, R. Y. Imagining imaging's future. Nature Rev. Mol. Cell Biol. (Suppl.), SS16–SS21 (2003).
Weissleder, R. et al. In vivo magnetic resonance imaging of transgene expression. Nature Med. 6, 351–355 (2000).
Higuchi, M. et al. 19F and 1H MRI detection of amyloid-β plaques in vivo. Nature Neurosci. 8, 527–533 (2005).
Oweida, A. J., Dunn, E. A. & Foster, P. J. Cellular imaging at 1.5 T: detecting cells in neuroinflammation using active labeling with superparamagnetic iron oxide. Mol. Imaging 3, 85–95 (2004).
Stettler, D. D., Yamahachi, H., Li, W., Denk, W. & Gilbert, C. D. Axons and synaptic boutons are highly dynamic in adult visual cortex. Neuron 49, 877–887 (2006).
Hell, S. W. Toward fluorescence nanoscopy. Nature Biotechnol. 21, 1347–1355 (2003).
Hell, S. W., Dyba, M. & Jakobs, S. Concepts for nanoscale resolution in fluorescence microscopy. Curr. Opin. Neurobiol. 14, 599–609 (2004). References 159 and 160 provide a stimulating introduction to recent technological developments towards fluorescence imaging beyond the diffraction limit.
Kleinfeld, D. & Griesbeck, O. From art to engineering? The rise of in vivo mammalian electrophysiology via genetically targeted labeling and nonlinear imaging. PLoS Biol. 3, e355 (2005).
Nguyen, Q. T., Callamaras, N., Hsieh, C. & Parker, I. Construction of a two-photon microscope for video-rate Ca2+ imaging. Cell Calcium 30, 383–393 (2001).
Majewska, A., Yiu, G. & Yuste, R. A custom-made two-photon microscope and deconvolution system. Pflugers Arch. 441, 398–408 (2000).
Tsai, P. S. et al. All-optical histology using ultrashort laser pulses. Neuron 39, 27–41 (2003).
Lichtman, J. W., Magrassi, L. & Purves, D. Visualization of neuromuscular junctions over periods of several months in living mice. J. Neurosci. 7, 1215–1222 (1987).
Sawamoto, K. et al. Visualization, direct isolation, and transplantation of midbrain dopaminergic neurons. Proc. Natl Acad. Sci. USA 98, 6423–6428 (2001).
Gong, S. et al. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature 425, 917–925 (2003).
Tanaka, D., Nakaya, Y., Yanagawa, Y., Obata, K. & Murakami, F. Multimodal tangential migration of neocortical GABAergic neurons independent of GPI-anchored proteins. Development 130, 5803–5813 (2003).
Araki, R. et al. Transgenic mouse lines expressing synaptopHluorin in hippocampus and cerebellar cortex. Genesis 42, 53–60 (2005).
Duebel, J. et al. Two-photon imaging reveals somatodendritic chloride gradient in retinal ON-type bipolar cells expressing the biosensor Clomeleon. Neuron 49, 81–94 (2006).
Mallon, B. S., Shick, H. E., Kidd, G. J. & Macklin, W. B. Proteolipid promoter activity distinguishes two populations of NG2-positive cells throughout neonatal cortical development. J. Neurosci. 22, 876–885 (2002).
Motoike, T. et al. Universal GFP reporter for the study of vascular development. Genesis 28, 75–81 (2000).
Acknowledgements
We wish to express our gratitude to J. Lichtman for his mentorship and support. Our thanks also go to J. Sanes, H. Wekerle and R. Hohlfeld for continued support. J. Lichtman, R. Hohlfeld and L. Godinho made valuable suggestions after reading a previous version of this manuscript. Work in our laboratories is supported by the Christopher Reeve Paralysis Foundation, the 'Brain-Immune Imaging Program' of the Dana-Foundation, the 'Emmy-Noether-Program' of the Deutsche Forschungsgemeinschaft, and the 'Verein Therapieforschung für MS-Kranke e.V.' We acknowledge B. Engelhardt (University of Bern, Switzerland), D. Kleinfeld (University of California, San Diego, USA) and B. Hyman (Harvard Medical School, Boston, USA) for permission to reproduce figure material from their work, and apologize to colleagues whose work we had no space to cover.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Optical sectioning
-
The exclusion of signal from out-of-focus planes. In confocal microscopy this is achieved by placing a pinhole in front of the detector. In multiphoton microscopy, optical sectioning is an intrinsic property, as excitation is limited to the perifocal volume, where photon density peaks.
- Diffraction limit
-
Fundamental limitation of the resolution achievable with conventional light microscopy. As a result of light diffraction, the focal 'spot' cannot be made smaller than 100–200 nm in the plane of focus and 0.5 μm in depth (depending on the objective, immersion media and wavelength).
- Charge-coupled device cameras
-
(CCD cameras). Digital cameras used for wide-field microscopy that incorporate silicon chips as detectors; light that hits the photo-sensitive areas of the chip generates charge, which is measured to generate a digital image.
- Photomultiplier tubes
-
Point detectors used in many scanning microscopes (for example, confocal laser scanning or multiphoton microscopes), which have a photocathode as a front surface that releases electrons on illumination, and a secondary electrode array that multiplies the signal before readout.
- Perifocal volume
-
The volume surrounding the focus of an objective lens; real lenses do not focus light into a geometrical 'point' but into a small spheroid volume, the size of which depends on the wavelength of the light and the characteristics of the objective.
- Phototoxicity
-
Toxic effects of light on cells that limits most in vivo microscopy experiments; caused at least in part by bleaching and associated photochemical generation of free radicals.
- XFP
-
Green fluorescent proteins or coral fluorescent proteins and their spectral variants, such as yellow and cyan fluorescent proteins.
- Knock-in strategy
-
Technique by which an endogenous gene is replaced with a novel sequence; for example, to inactivate the gene and/or to characterize its expression pattern. Knock in strategies differ from 'transgenic' approaches in which genetic material is randomly inserted into the genome.
- Vital dye
-
Used in microscopy to denote any compound that can be applied to living organisms and cells to stain specific structures.
- Intrinsic contrast
-
All features of normal tissue that allow the detection of structures without additional labelling. Techniques that use intrinsic contrast are phase contrast microscopy and skeletal X-rays.
- Multicell bolus loading
-
Recent innovation in the field of calcium imaging in which large groups of neurons or glial cells are loaded with calcium indicator dyes through the injection of concentrated solutions of cell-permeant dye esters into brain tissue.
- Fluorescence resonance energy transfer
-
(FRET). Non-radiative transfer of photons between molecules with overlapping excitation–emission spectra; depends strongly on the proximity of molecules and can therefore indicate molecular interactions.
- Transcranial imaging
-
In the context of in vivo microscopy, this refers to imaging through the skull; multiphoton imaging can image 'through' thin slivers of bone, so that a craniotomy can be avoided by drilling away all but the inner table of the skull.
- Negative contrast
-
Form of contrast in which the object of interest is not labelled but surrounded by stained 'background'— for example, an unlabelled erythrocyte in fluorescently labelled serum.
- Photothrombotic stroke
-
Form of small vessel occlusion that depends on 'photosensitizers' — that is, molecules like rose bengal that cause vessel damage in response to being illuminated. By injecting such molecules intravenously, local clotting ('thrombosis') can be targeted by light ('optical' micro-occlusion models).
- Micro-embolic stroke
-
Miniature stroke model that is induced by injecting small particles into the blood stream. These particles lodge in microvessels and occlude them ('emboli'). This model can be acheived by injecting microspheres or fragmented clots into a major artery.
- Tomographic approaches
-
Describes imaging approaches in which the object is imaged from multiple angles and mathematical algorithms are used to reconstruct the three-dimensional structure of the signal source.
- Bioluminescence resonant energy transfer
-
(BRET). Energy transfer in which the donor is a bioluminescent molecule that emits light as part of a biochemical reaction and transfers it to a fluorescent acceptor. Green fluorescent protein is the acceptor in a BRET cascade that makes some jellyfish glow.
- Interferometry
-
Optical tool used to measure differences in path length or travel time of light by interference — that is, the addition or cancellation of light waves as a consequence of phase shifts.
- Contrast agent
-
Used in clinical context for agents that generate a strong signal in an imaging technique and can be injected into the blood stream, swallowed by a patient or introduced into a body cavity.
- Bimodal contrast agent
-
A compound that enhances contrast in more than one imaging modality, for example, a substance that generates an MRI signal and is fluorescent at the same time.
Rights and permissions
About this article
Cite this article
Misgeld, T., Kerschensteiner, M. In vivo imaging of the diseased nervous system. Nat Rev Neurosci 7, 449–463 (2006). https://doi.org/10.1038/nrn1905
Issue Date:
DOI: https://doi.org/10.1038/nrn1905
This article is cited by
-
Neuro-nanotechnology: diagnostic and therapeutic nano-based strategies in applied neuroscience
BioMedical Engineering OnLine (2023)
-
In vivo imaging of injured cortical axons reveals a rapid onset form of Wallerian degeneration
BMC Biology (2020)
-
Subcellular spatial resolution achieved for deep-brain imaging in vivo using a minimally invasive multimode fiber
Light: Science & Applications (2018)
-
Spatiotemporal changes of optical signals in the somatosensory cortex of neuropathic rats after electroacupuncture stimulation
BMC Complementary and Alternative Medicine (2017)
-
Vectorized nanodelivery systems for ischemic stroke: a concept and a need
Journal of Nanobiotechnology (2017)