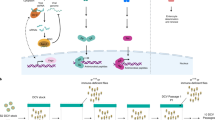
Key Points
-
This Review describes the crosstalk that exists between bacteria and insects, and, particularly, discusses the strategies that are used by bacterial pathogens to persist in insects and the responses of insects to such infections.
-
Insect infections can occur through different routes: bacteria can reach the haemolymph after wounding or assisted entry, but most of the interactions between insects and bacteria happen upon ingestion of contaminated food.
-
Only a handful of bacteria can persist in large numbers in the digestive tracts of insects. It is generally assumed that most ingested bacteria are eliminated from the gut environment by peristalsis or by other unknown mechanisms. The ability of bacteria to persist can be due to a single gene, and might be related to perturbation of gut physiology.
-
Bacteria that can persist after ingestion must overcome the insect immune response. The insect immune response occurs at the infection site in the gut, but some persisting bacteria can also trigger antimicrobial peptide production at the systemic level. Some entomopathogenic bacteria can evade the insect immune response, mainly by suppressing it.
-
Despite the characterization of multiple virulence factors, how pathogens kill insects is not known. Insect death results either from bacterial proliferation or from damages that are caused by a toxic factor (or factors). Multiple factors have been identified, and many of them are under the control of global regulatory systems.
-
In conclusion, it is becoming evident that bacterial infections of insect hosts involve crosstalk that is similar to the crosstalk that is required for bacterial infections of other metazoan hosts. The acquisition of determinants that allow bacteria to persist, or to counteract insect host defences, might change the host range of pathogens.
Abstract
Recent genetic and molecular analyses have revealed how several strategies enable bacteria to persist and overcome insect immune defences. Genetic and genomic tools that can be used with Drosophila melanogaster have enabled the characterization of the pathways that are used by insects to detect bacterial invaders and combat infection. Conservation of bacterial virulence factors and insect immune repertoires indicates that there are common strategies of host invasion and pathogen eradication. Long-term interactions of bacteria with insects might ensure efficient dissemination of pathogens to other hosts, including humans.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Boucias, D. G. & Pendland, J. C. Principles of Insect Pathology (Kluwer Academic Publishers, Dordrecht, 1998).
Federici, B. A., Park, H. W., Bideshi, D. K., Wirth, M. C. & Johnson, J. J. Recombinant bacteria for mosquito control. J. Exp. Biol. 206, 3877–3885 (2003).
Aballay, A. & Ausubel, F. M. Caenorhabditis elegans as a host for the study of host–pathogen interactions. Curr. Opin. Microbiol. 5, 97–101 (2002).
Cosson, P. et al. Pseudomonas aeruginosa virulence analyzed in a Dictyostelium discoideum host system. J. Bacteriol. 184, 3027–3033 (2002).
Rahme, L. G. et al. Use of model plant hosts to identify Pseudomonas aeruginosa virulence factors. Proc. Natl Acad. Sci. USA 94, 13245–13250 (1997).
Vodovar, N., Acosta, C., Lemaitre, B. & Boccard, F. Drosophila: a polyvalent model to decipher host–pathogen interactions. Trends Microbiol. 12, 235–242 (2004).
Clarkson, J. M. & Charnley, A. K. New insights into the mechanisms of fungal pathogenesis in insects. Trends Microbiol. 4, 197–203 (1996).
Baumann, P. & Moran, N. A. Non-cultivable microorganisms from symbiotic associations of insects and other hosts. Antonie Van Leeuwenhoek 72, 39–48 (1997).
Gil, R., Latorre, A. & Moya, A. Bacterial endosymbionts of insects: insights from comparative genomics. Environ. Microbiol. 6, 1109–1122 (2004).
Bulla, L. A., R. A., R. & St. Julian, G. Bacteria as insect pathogens. Annu. Rev. Microbiol. 29, 163–190 (1975).
Lysenko, O. Non-sporeforming bacteria pathogenic to insects: incidence and mechanisms. Annu. Rev. Microbiol. 39, 673–695 (1985).
Kobayashi, M. et al. Houseflies: not simple mechanical vectors of enterohemorrhagic Escherichia coli O157:H7. Am. J. Trop. Med. Hyg. 61, 625–629 (1999).
Douglas, A. E. & Beard, C. B. in Biology of the Insect Midgut (eds Lehane, M. J. & Billingsley, P. F.) (Chapman & Hall, London, 1996).
Kaslow, D. C. & Welburn, S. in Biology of the Insect Midgut (eds Lehane, M. J. & Billingsley, P. F.) (Chapman & Hall, London, 1996).
Pai, H. H., Chen, W. C. & Peng, C. F. Cockroaches as potential vectors of nosocomial infections. Infect. Control Hosp. Epidemiol. 25, 979–984 (2004).
Gage, K. L. & Kosoy, M. Y. Natural history of plague: perspectives from more than a century of research. Annu. Rev. Entomol. 50, 505–528 (2005).
Chapman, R. F. The Insects: Structure and Function (Cambridge University Press, Cambridge, 1998).
Tzou, P. et al. Tissue-specific inducible expression of antimicrobial peptide genes in Drosophila surface epithelia. Immunity 13, 737–748 (2000).
Hao, Z., Kasumba, I. & Aksoy, S. Proventriculus (cardia) plays a crucial role in immunity in tsetse fly (Diptera: Glossinidiae). Insect Biochem. Mol. Biol. 33, 1155–1164 (2003).
Jarrett, C. O. et al. Transmission of Yersinia pestis from an infectious biofilm in the flea vector. J. Infect. Dis. 190, 783–792 (2004).
Ha, E. M., Oh, C. T., Bae, Y. S. & Lee, W. J. A direct role for dual oxidase in Drosophila gut immunity. Science 310, 847–850 (2005). Shows that dual oxidase is indispensable for gut antimicrobial activities in adult Drosophila.
Ha, E. M. et al. An antioxidant system required for host protection against gut infection in Drosophila. Dev. Cell 8, 125–132 (2005). Shows that homeostasis in redox balance modulates host survival after ingestion of bacteria.
Hinnebusch, B. J. et al. Role of Yersinia murine toxin in survival of Yersinia pestis in the midgut of the flea vector. Science 296, 733–735 (2002). Identification of the gene that is responsible for gut persistence in fleas.
Perry, R. D., Pendrak, M. L. & Schuetze, P. Identification and cloning of a hemin storage locus involved in the pigmentation phenotype of Yersinia pestis. J. Bacteriol. 172, 5929–5937 (1990).
Parsek, M. R. & Singh, P. K. Bacterial biofilms: an emerging link to disease pathogenesis. Annu. Rev. Microbiol. 57, 677–701 (2003).
Hinnebusch, B. J., Perry, R. D. & Schwan, T. G. Role of the Yersinia pestis hemin storage (hms) locus in the transmission of plague by fleas. Science 273, 367–370 (1996). Identification of the gene responsible for proventriculus colonization and digestive tract blockage.
Darby, C., Ananth, S. L., Tan, L. & Hinnebusch, B. J. Identification of gmhA, a Yersinia pestis gene required for flea blockage, by using a Caenorhabditis elegans biofilm system. Infect. Immun. 73, 7236–7242 (2005).
Flyg, C., Kenne, K. & Boman, H. G. Insect pathogenic properties of Serratia marcescens: phage-resistant mutants with a decreased resistance to Cecropia immunity and a decreased virulence to Drosophila. J. Gen. Microbiol. 120, 173–181 (1980).
Chugani, S. A. et al. QscR, a modulator of quorum-sensing signal synthesis and virulence in Pseudomonas aeruginosa. Proc. Natl Acad. Sci. USA 98, 2752–2757 (2001).
Basset, A. et al. The phytopathogenic bacteria Erwinia carotovora infects Drosophila and activates an immune response. Proc. Natl Acad. Sci. USA 97, 3376–3381 (2000). First report of immune-response activation after ingestion of bacteria by Drosophila larvae.
Vodovar, N. et al. Drosophila host defense after oral infection by an entomopathogenic Pseudomonas species. Proc. Natl Acad. Sci. USA 102, 11414–11419 (2005). Characterization of P. entomophila pathogenicity in Drosophila.
Blow, N. S. et al. Vibrio cholerae infection of Drosophila melanogaster mimics the human disease cholera. PLoS Pathog. 1, e8 (2005).
Nehme, N. T. et al. A model of bacterial intestinal infections in Drosophila melanogaster. PLoS Pathog. 3, e173 (2007). Characterization of S. marcescens pathogenicity in Drosophila.
Kloepper, J. W., Brewer, J. W. & Harisson, M. D. Insect transmission of Erwinia carotovora var. carotovora and Erwinia carotovora var. atroseptica to potato plants in the field. Am. Potato. J. 58, 165–175 (1981).
Molina, J. J., Harisson, M. D. & Brewer, J. W. Transmission of Erwinia carotovora var. atroseptica by Drosophila melanogaster meig. I. Acquisition and transmission of the bacterium. Am. Potato J. 54, 245–250 (1974).
Basset, A., Tzou, P., Lemaitre, B. & Boccard, F. A single gene that promotes interaction of a phytopathogenic bacterium with its insect vector, Drosophila melanogaster. EMBO Rep. 4, 205–209 (2003). Identification of the gene that allows bacterial persistence in the Drosophila larval gut.
Acosta Muniz, C., Jaillard, D., Lemaitre, B. & Boccard, F. Erwinia carotovora Evf antagonizes the elimination of bacteria in the gut of Drosophila larvae. Cell. Microbiol. 9, 106–119 (2007).
Jackson, T. A., Boucias, D. G. & Thaler, J. O. Pathobiology of amber disease, caused by Serratia spp., in the New Zealand grass grub, Costelytra zealandica. J. Invertebr. Pathol. 78, 232–243 (2001).
Hurst, M. R. & Jackson, T. A. Use of the green fluorescent protein to monitor the fate of Serratia entomophila causing amber disease in the New Zealand grass grub, Costelytra zealandica. J. Microbiol. Methods 50, 1–8 (2002).
Hurst, M. R., Glare, T. R., Jackson, T. A. & Ronson, C. W. Plasmid-located pathogenicity determinants of Serratia entomophila, the causal agent of amber disease of grass grub, show similarity to the insecticidal toxins of Photorhabdus luminescens. J. Bacteriol. 182, 5127–5138 (2000). Identification of determinants responsible for Amber disease.
Grkovic, S., Glare, T. R., Jackson, T. A. & Corbett, G. E. Genes essential for amber disease in grass grubs are located on the large plasmid found in Serratia entomophila and Serratia proteamaculans. Appl. Environ. Microbiol. 61, 2218–2223 (1995).
Hurst, M. R., Glare, T. R. & Jackson, T. A. Cloning Serratia entomophila antifeeding genes — a putative defective prophage active against the grass grub Costelytra zealandica. J. Bacteriol. 186, 5116–5128 (2004).
Hurst, M. R., Beard, S. S., Jackson, T. A. & Jones, S. M. Isolation and characterization of the Serratia entomophila antifeeding prophage. FEMS Microbiol. Lett. 270, 42–48 (2007).
ffrench-Constant, R. H., Dowling, A. & Waterfield, N. R. Insecticidal toxins from Photorhabdus bacteria and their potential use in agriculture. Toxicon 49, 436–451 (2007).
Onfelt Tingvall, T., Roos, E. & Engstrom, Y. The imd gene is required for local Cecropin expression in Drosophila barrier epithelia. EMBO Rep. 2, 239–243 (2001).
Zaidman-Remy, A. et al. The Drosophila amidase PGRP-LB modulates the immune response to bacterial infection. Immunity 24, 463–473 (2006).
Bischoff, V. et al. Downregulation of the Drosophila immune response by peptidoglycan-recognition proteins SC1 and SC2. PLoS Pathog. 2, e14 (2006).
Ryu, J. H. et al. An essential complementary role of NF-κB pathway to microbicidal oxidants in Drosophila gut immunity. EMBO J. 25, 3693–3701 (2006). Shows that intestinal NF-κB- and AMP-dependent immunity is crucial to host survival against ROS-resistant bacteria.
Liehl, P., Blight, M., Vodovar, N., Boccard, F. & Lemaitre, B. Prevalence of local immune response against oral infection in a Drosophila/Pseudomonas infection model. PLoS Pathog. 2, e56 (2006). Shows the importance of the bacterial metalloprotease AprA to counteract AMPs.
Vodovar, N. et al. Complete genome sequence of the entomopathogenic and metabolically versatile soil bacterium Pseudomonas entomophila. Nature Biotechnol. 24, 673–679 (2006).
Leulier, F. et al. The Drosophila immune system detects bacteria through specific peptidoglycan recognition. Nature Immunol. 4, 478–484 (2003).
Royet, J. & Dziarski, R. Peptidoglycan recognition proteins: pleiotropic sensors and effectors of antimicrobial defences. Nature Rev. Microbiol. 5, 264–277 (2007).
Forst, S., Dowds, B., Boemare, N. & Stackebrandt, E. Xenorhabdus and Photorhabdus spp.: bugs that kill bugs. Annu. Rev. Microbiol. 51, 47–72 (1997).
Goodrich-Blair, H. & Clarke, D. J. Mutualism and pathogenesis in Xenorhabdus and Photorhabdus: two roads to the same destination. Mol. Microbiol. 64, 260–268 (2007).
Hurst, G. D., Anbutsu, H., Kutsukake, M. & Fukatsu, T. Hidden from the host: Spiroplasma bacteria infecting Drosophila do not cause an immune response, but are suppressed by ectopic immune activation. Insect Mol. Biol. 12, 93–97 (2003). Host colonization by bacteria that avoid detection by the insect defences.
Herbert, E. E. & Goodrich-Blair, H. Friend and foe: the two faces of Xenorhabdus nematophila. Nature Rev. Microbiol. 5, 634–646 (2007).
Ji, D. & Kim, Y. An entomopathogenic bacterium, Xenorhabdus nematophila, inhibits the expression of an antibacterial peptide, cecropin, of the beet armyworm, Spodoptera exigua. J. Insect Physiol. 50, 489–496 (2004).
Apidianakis, Y. et al. Profiling early infection responses: Pseudomonas aeruginosa eludes host defenses by suppressing antimicrobial peptide gene expression. Proc. Natl Acad. Sci. USA 102, 2573–2578 (2005).
Park, Y., Kim, Y., Putnam, S. M. & Stanley, D. W. The bacterium Xenorhabdus nematophilus depresses nodulation reactions to infection by inhibiting eicosanoid biosynthesis in tobacco hornworms, Manduca sexta. Arch. Insect Biochem. Physiol. 52, 71–80 (2003).
Kim, Y., Ji, D., Cho, S. & Park, Y. Two groups of entomopathogenic bacteria, Photorhabdus and Xenorhabdus, share an inhibitory action against phospholipase A2 to induce host immunodepression. J. Invertebr. Pathol. 89, 258–264 (2005).
Eleftherianos, I., Millichap, P. J., ffrench-Constant, R. H. & Reynolds, S. E. RNAi suppression of recognition protein mediated immune responses in the tobacco hornworm Manduca sexta causes increased susceptibility to the insect pathogen Photorhabdus. Dev. Comp. Immunol. 30, 1099–1107 (2006). A small antibiotic molecule that is produced by Photorhabdus spp. acts as inhibitor of phenoloxidase in Manduca sexta.
Brugirard-Ricaud, K. et al. Site-specific antiphagocytic function of the Photorhabdus luminescens type III secretion system during insect colonization. Cell. Microbiol. 7, 363–371 (2005).
Eleftherianos, I. et al. An antibiotic produced by an insect-pathogenic bacterium suppresses host defenses through phenoloxidase inhibition. Proc. Natl Acad. Sci. USA 104, 2419–2424 (2007).
Haas, D. & Defago, G. Biological control of soil-borne pathogens by fluorescent pseudomonads. Nature Rev. Microbiol. 3, 307–319 (2005).
Galan, J. E. & Collmer, A. Type III secretion machines: bacterial devices for protein delivery into host cells. Science 284, 1322–1328 (1999).
Matsumoto, K. Role of bacterial proteases in pseudomonal and serratial keratitis. Biol. Chem. 385, 1007–1016 (2004).
Miyoshi, S. & Shinoda, S. Microbial metalloproteases and pathogenesis. Microbes Infect. 2, 91–98 (2000).
Travis, J., Potempa, J. & Maeda, H. Are bacterial proteinases pathogenic factors? Trends Microbiol. 3, 405–407 (1995).
ffrench-Constant, R. et al. Photorhabdus: towards a functional genomic analysis of a symbiont and pathogen. FEMS Microbiol. Rev. 26, 433–456 (2003).
Bowen, D. J. et al. Genetic and biochemical characterization of PrtA, an RTX-like metalloprotease from Photorhabdus. Microbiology 149, 1581–1591 (2003).
Duchaud, E. et al. The genome sequence of the entomopathogenic bacterium Photorhabdus luminescens. Nature Biotechnol. 21, 1307–1313 (2003).
Derzelle, S. et al. The PhoP/PhoQ two-component regulatory system of Photorhabdus luminescens is essential for virulence in insects. J. Bacteriol. 186, 1270–1279 (2004).
Cowles, K. N., Cowles, C. E., Richards, G. R., Martens, E. C. & Goodrich-Blair, H. The global regulator Lrp contributes to mutualism, pathogenesis and phenotypic variation in the bacterium Xenorhabdus nematophila. Cell. Microbiol. 9, 1311–1323 (2007).
Fedhila, S., Nel, P. & Lereclus, D. The InhA2 metalloprotease of Bacillus thuringiensis strain 407 is required for pathogenicity in insects infected via the oral route. J. Bacteriol. 184, 3296–3304 (2002). Demonstrates the involvement of metalloprotease in the pathogenesis of B. thuringiensis.
Salamitou, S. et al. The plcR regulon is involved in the opportunistic properties of Bacillus thuringiensis and Bacillus cereus in mice and insects. Microbiology 146, 2825–2832 (2000).
Titball, R. W., Hill, J., Lawton, D. G. & Brown, K. A. Yersinia pestis and plague. Biochem. Soc. Trans. 31, 104–107 (2003).
Waterfield, N. R., Wren, B. W. & Ffrench-Constant, R. H. Invertebrates as a source of emerging human pathogens. Nature Rev. Microbiol. 2, 833–841 (2004).
Grenier, A. M., Duport, G., Pages, S., Condemine, G. & Rahbe, Y. The phytopathogen Dickeya dadantii (Erwinia chrysanthemi 3937) is a pathogen of the pea aphid. Appl. Environ. Microbiol. 72, 1956–1965 (2006).
Dillon, R. J. & Dillon, V. M. The gut bacteria of insects: nonpathogenic interactions. Annu. Rev. Entomol. 49, 71–92 (2004).
Eleftherianos, I. et al. Prior infection of Manduca sexta with non-pathogenic Escherichia coli elicits immunity to pathogenic Photorhabdus luminescens: roles of immune-related proteins shown by RNA interference. Insect Biochem. Mol. Biol. 36, 517–525 (2006).
Tzou, P., Reichhart, J. M. & Lemaitre, B. Constitutive expression of a single antimicrobial peptide can restore wild-type resistance to infection in immunodeficient Drosophila mutants. Proc. Natl Acad. Sci. USA 99, 2152–2157 (2002).
Dimopoulos, G. Insect immunity and its implication in mosquito-malaria interactions. Cell. Microbiol. 5, 3–14 (2003).
Evans, J. D. et al. Immune pathways and defence mechanisms in honey bees Apis mellifera. Insect Mol. Biol. 15, 645–656 (2006).
Zou, Z. et al. Comparative genomic analysis of the Tribolium immune system. Genome Biol. 8, R177 (2007).
Kanost, M. R., Jiang, H. & Yu, X. Q. Innate immune responses of a lepidopteran insect, Manduca sexta. Immunol. Rev. 198, 97–105 (2004).
Ryu, J. H. et al. Innate immune homeostasis by the homeobox gene caudal and commensal-gut mutualism in Drosophila. Science 319, 777–782 (2008). Describes changes in the commensal community by modification of host innate immune homeostasis and protection from ingested pathogens by the microbiota.
Shirasu-Hiza, M. M. & Schneider, D. S. Confronting physiology: how do infected flies die? Cell. Microbiol. 9, 2775–2783 (2007). Characterization of the colonization process of Naucoris by M. ulcerans.
Marsollier, L. et al. Protection against Mycobacterium ulcerans lesion development by exposure to aquatic insect saliva. PLoS Med. 4, e64 (2007).
Marsollier, L. et al. Colonization of the salivary glands of Naucoris cimicoides by Mycobacterium ulcerans requires host plasmatocytes and a macrolide toxin, mycolactone. Cell. Microbiol. 7, 935–943 (2005).
Marsollier, L. et al. Aquatic insects as a vector for Mycobacterium ulcerans. Appl. Environ. Microbiol. 68, 4623–4628 (2002).
Marsollier, L. et al. Impact of Mycobacterium ulcerans biofilm on transmissibility to ecological niches and Buruli ulcer pathogenesis. PLoS Pathog. 3, e62 (2007).
Brennan, C. A. & Anderson, K. V. Drosophila: the genetics of innate immune recognition and response. Annu. Rev. Immunol. 22, 457–483 (2004).
Lemaitre, B. & Hoffmann, J. The host defense of Drosophila melanogaster. Annu. Rev. Immunol. 25, 697–743 (2007).
Foley, E. & O'Farrell, P. H. Nitric oxide contributes to induction of innate immune responses to Gram-negative bacteria in Drosophila. Genes Dev. 17, 115–125 (2003).
Dijkers, P. & O'Farrell, P. H. Drosophila calcineurin promotes induction of innate immune responses. Curr. Biol. 17, 2087–2093 (2007).
Uvell, H. & Engstrom, Y. A multilayered defense against infection: combinatorial control of insect immune genes. Trends Genet. 23, 342–349 (2007).
Kocks, C. et al. Eater, a transmembrane protein mediating phagocytosis of bacterial pathogens in Drosophila. Cell 123, 335–346 (2005).
Cerenius, L. & Soderhall, K. The prophenoloxidase-activating system in invertebrates. Immunol. Rev. 198, 116–126 (2004).
Gillespie, J. P., Kanost, M. R. & Trenczek, T. Biological mediators of insect immunity. Annu. Rev. Entomol. 42, 611–643 (1997).
Hurst, G. D. & Jiggins, F. M. Male-killing bacteria in insects: mechanisms, incidence, and implications. Emerg. Infect. Dis. 6, 329–336 (2000).
McGraw, E. A. & O'Neill, S. L. Wolbachia pipientis: intracellular infection and pathogenesis in Drosophila. Curr. Opin. Microbiol. 7, 67–70 (2004).
Dobson, S. L. et al. Wolbachia infections are distributed throughout insect somatic and germ line tissues. Insect Biochem. Mol. Biol. 29, 153–160 (1999).
Bourtzis, K., Pettigrew, M. M. & O'Neill, S. L. Wolbachia neither induces nor suppresses transcripts encoding antimicrobial peptides. Insect Mol. Biol. 9, 635–639 (2000).
Min., K. T. & Benzer, S. Wolbachia, normally a symbiont of Drosophila, can be virulent, causing degeneration and early death. Proc. Natl Acad. Sci. USA 94, 10792–10796 (1997).
Priest, F. G. in Entomopathogenic Bacteria: From Laboratory to Field Application (eds Charles, J.-F., Delécluse, A. & Nielsen-LeRoux, C.) 1–22 (Kluwer Academic Publishers, Dordrecht, 2000).
Bravo, A., Gill, S. S. & Soberon, M. Mode of action of Bacillus thuringiensis Cry and Cyt toxins and their potential for insect control. Toxicon 49, 423–435 (2007).
de Maagd, R. A., Bravo, A., Berry, C., Crickmore, N. & Schnepf, H. E. Structure, diversity, and evolution of protein toxins from spore-forming entomopathogenic bacteria. Annu. Rev. Genet. 37, 409–433 (2003).
Schnepf, E. et al. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol. Mol. Biol. Rev. 62, 775–806 (1998).
Aronson, A. I. & Shai, Y. Why Bacillus thuringiensis insecticidal toxins are so effective: unique features of their mode of action. FEMS Microbiol. Lett. 195, 1–8 (2001).
Peferoen, M. Progress and prospects for field use of Bt genes in crops. TIBTech, 173–177 (1997).
Hansen, B. M. & Salamitou, S. in Entomopathogenic Bacteria: From Laboratory to Field Application (eds Charles, J.-F., Delécluse, A. & Nielsen-LeRoux, C.) 41–64 (Kluwer Academic Publishers, Dordrecht, 2000).
Broderick, N. A., Raffa, K. F. & Handelsman, J. Midgut bacteria required for Bacillus thuringiensis insecticidal activity. Proc. Natl Acad. Sci. USA 103, 15196–15199 (2006). Demonstrates that commensal bacteria are required to kill gypsy moth upon B. thuringiensis infection.
Harada, H. & Ishikawa, H. Experimental pathogenicity of Erwinia aphidicola to pea aphid, Acyrthosiphon pisum. J. Gen. Appl. Microbiol. 43, 363–367 (1997).
Fedhila, S., Daou, N., Lereclus, D. & Nielsen-LeRoux, C. Identification of Bacillus cereus internalin and other candidate virulence genes specifically induced during oral infection in insects. Mol. Microbiol. 62, 339–355 (2006).
Rolain, J. M., Franc, M., Davoust, B. & Raoult, D. Molecular detection of Bartonella quintana, B. koehlerae, B. henselae, B. clarridgeiae, Rickettsia felis, and Wolbachia pipientis in cat fleas, France. Emerg. Infect. Dis. 9, 338–342 (2003).
Bowen, D. et al. Insecticidal toxins from the bacterium Photorhabdus luminescens. Science 280, 2129–2132 (1998). Identification of Tc-toxins.
Morgan, J. A., Sergeant, M., Ellis, D., Ousley, M. & Jarrett, P. Sequence analysis of insecticidal genes from Xenorhabdus nematophilus PMFI296. Appl. Environ. Microbiol. 67, 2062–2069 (2001).
Waterfield, N. R., Bowen, D. J., Fetherston, J. D., Perry, R. D. & ffrench-Constant, R. H. The tc genes of Photorhabdus: a growing family. Trends Microbiol. 9, 185–191 (2001).
Charles, J. F., Silva-Filha, M.-H. & Nielsen-LeRoux, C. in Entomopathogenic Bacteria: From Laboratory to Field Application (eds Charles, J. F., Delécluse, A. & Nielsen-LeRoux, C.) 237–252 (Kluwer Academic Press, Dordrecht, 2000).
Daborn, P. J. et al. A single Photorhabdus gene, makes caterpillars floppy (mcf), allows Escherichia coli to persist within and kill insects. Proc. Natl Acad. Sci. USA 99, 10742–10747 (2002). Identification of the Mcf toxin.
Acknowledgements
The authors thank F. Leulier, N. Vodovar and C. Nielsen-LeRoux for helpful discussions and critical reading of the manuscript. Work described in this Review that was performed in our laboratories was supported by Centre National de la Recherche Scientifique (CNRS), Agence Nationale de la Recherche, the Schlumberger and Bettancourt Foundations, and the Association 'Vaincre la Mucoviscidose'.
Author information
Authors and Affiliations
Corresponding author
Related links
Related links
DATABASES
Entrez Genome Project
FURTHER INFORMATION
Glossary
- Persistence
-
The survival of bacteria in large numbers in a host.
- Colonization
-
The ability to multiply in the host.
- Innate immunity
-
Effector mechanisms that control infection and that possess a certain degree of specificity to different classes of microorganisms.
- Biofilm
-
Association of bacteria to form a structured community, in contrast to free-living, planktonic bacteria.
- Pathogenic
-
The ability to cause damage to a host.
- Tc-toxin
-
Insecticidal toxin complex, originally characterized in Photorhabdus and Xenorhabdus species.
- Peptidoglycan recognition proteins
-
Peptidoglycan recognition proteins are innate immunity molecules that are present in most invertebrate and vertebrate animals.
- Infectious
-
The ability to colonize a host and induce the host immune response.
- Type III secretion system
-
A secretion apparatus that allows direct injection of effectors from bacteria into host cells.
Rights and permissions
About this article
Cite this article
Vallet-Gely, I., Lemaitre, B. & Boccard, F. Bacterial strategies to overcome insect defences. Nat Rev Microbiol 6, 302–313 (2008). https://doi.org/10.1038/nrmicro1870
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrmicro1870
This article is cited by
-
Rodents as vehicle for delivery of transgenic bacteria to make paratransgenic sand fly vectors of cutaneous leishmaniasis in field condition
Scientific Reports (2023)
-
Synergistic and additive interactions of Shewanella sp., Pseudomonas sp. and Thauera sp. with chlorantraniliprole and emamectin benzoate for controlling Spodoptera litura (Fabricius)
Scientific Reports (2023)
-
Relation of pest insect-killing and soilborne pathogen-inhibition abilities to species diversification in environmental Pseudomonas protegens
The ISME Journal (2023)
-
A fat body transcriptome analysis of the immune responses of Rhodnius prolixus to artificial infections with bacteria
Parasites & Vectors (2022)
-
Lignocellulose degradation in Protaetia brevitarsis larvae digestive tract: refining on a tightly designed microbial fermentation production line
Microbiome (2022)