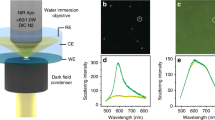
Abstract
Heterogeneous catalysts have been pivotal to the development of the modern chemical industry and are essential for catalysing many industrial reactions. However, reaction rates are different for every individual catalyst particle and depend upon each particle's morphology and size1, crystal structure2 and composition3,4,5,6,7. Measuring the rates of reaction on single nanocrystals will enable the role of catalyst structure to be quantified. Here, using surface plasmon spectroscopy, we have directly observed the kinetics of atomic deposition onto a single gold nanocrystal and also monitored electron injection and extraction during a redox reaction involving the oxidation of ascorbic acid on a gold nanocrystal surface. These results constitute the first direct measurement of the rates of redox catalysis on single nanocrystals.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Li, Y. & El-Sayed, M. A. The effect of stabilizers on the catalytic activity and stability of Pd colloidal nanoparticles in the Suzuki reactions in aqueous solution. J. Phys. Chem. B 105, 8938–8943 (2001).
Meisel, D., Mulac, W. & Matheson, M. Catalysis of methyl viologen radical reactions by polymer-stabilized gold sols. J. Phys. Chem. 85, 179–187 (1981).
Narayanan, R. & El-Sayed, M. A. Shape-dependent catalytic activity of platinum nanoparticles in colloidal solution. Nano Lett. 4, 1343–1348 (2004).
Rupprechter, G. & Weilach, C. Mind the gap! Spectroscopy of catalytically active phases. Nano Today 2, 20–29 (2007).
Park, I., Lee, K., Choi, J., Park, H. & Sung, Y. Surface structure of Pt-modified Au nanoparticles and electrocatalytic activity in formic acid electro-oxidation. J. Phys. Chem. C 111, 19126–19133 (2007).
Chen, X. & Moskovits, M. Observing catalysis through the agency of the participating electrons: surface-chemistry-induced current changes in a tin oxide nanowire decorated with silver. Nano Lett. 7, 807–812 (2007).
Fierro-Gonzalez, J. & Gates, B. Evidence of active species in CO oxidation catalyzed by highly dispersed supported gold. Catal. Today 122, 201–210 (2007).
Somorjai, G., York, R., Butcher, D. & Park, J. The evolution of model catalytic systems: studies of structure, bonding and dynamics from single-crystal metal surfaces to nanoparticles, and from low pressure (<10−3 Torr) to high pressure (>10−3 Torr) to liquid interfaces. Phys. Chem. Chem. Phys. 9, 3500–3513 (2007).
Novo, C. et al. Contributions from radiation damping and surface scattering to the linewidth of the longitudinal plasmon band of gold nanorods: a single particle study. Phys. Chem. Chem. Phys. 8, 3540–3546 (2006).
Novo, C., Funston, A. M., Pastoriza-Santos, I., Liz-Marzán, L. & Mulvaney, P. Spectroscopy and high-resolution microscopy of single nanocrystals by a focused ion beam registration method. Angew. Chem. Int. Ed. 46, 3517–3520 (2007).
Henglein, A. Physicochemical properties of small metal particles in solution—microelectrode reactions, chemisorption, composite metal particles, and the atom-to-metal transition. J. Phys. Chem. 97, 5457–5471 (1993).
Bard, A., Crayston, J., Kittlesen, G., Shea, T. & Wrighton, M. Digital simulation of the measured electrochemical response of reversible redox couples at microelectrode arrays—consequences arising from closely spaced ultramicroelectrodes. Anal. Chem. 58, 2321–2331 (1986).
Mulvaney, P. Surface plasmon spectroscopy of nanosized metal particles. Langmuir 12, 788–800 (1996).
Bockris, J., Damjanov, A. & Mannan, R. Catalysis of electrodic hydrogen evolution and dissolution reactions on rationally chosen substrates. J. Electroanal. Chem. 18, 349–361 (1968).
Spiro, M. & Freund, P. Colloidal catalysis—transport versus surface control. J. Chem. Soc., Faraday Trans. I 79, 1649–1658 (1983).
Spiro, M. Heterogeneous catalysis in solution. 17. Kinetics of oxidation–reduction reactions catalyzed by electron-transfer through the solid—an electrochemical treatment. J. Chem. Soc., Faraday Trans. I 75, 1507–1512 (1979).
Henglein, A. & Lilie, J. Storage of electrons in aqueous solution—the rates of chemical charging and discharging the colloidal silver microelectrode. J. Am. Chem. Soc. 103, 1059–1066 (1981).
Miller, D., Bard, A., McLendon, G. & Ferguson, J. Catalytic water reduction at colloidal metal microelectrodes. 2. Theory and experiment. J. Am. Chem. Soc. 103, 5336–5341 (1981).
Kiwi, J. & Gratzel, M. Hydrogen evolution from water induced by visible light mediated by redox catalysis. Nature 281, 657–658 (1979).
Mulvaney, P., Perez-Juste, J., Giersig, M., Liz-Marzán, L. & Pecharroman, C. Drastic surface plasmon mode shifts in gold nanorods due to electron charging. Plasmonics 1, 61–66 (2006).
Novo, C. & Mulvaney, P. Charge-induced Rayleigh instabilities in small gold rods. Nano Lett. 7, 520–524 (2007).
Mulvaney, P., Linnert, T. & Henglein, A. Surface chemistry of colloidal silver in aqueous solution—observations on chemisorption and reactivity. J. Phys. Chem. 95, 7843–7846 (1991).
Liz-Marzán, L. Tailoring surface plasmons through the morphology and assembly of metal nanoparticles. Langmuir 22, 32–41 (2006).
Nikoobakht, B. & El-Sayed, M. A. Preparation and growth mechanism of gold nanorods (NRs) using seed-mediated growth method. Chem. Mater. 15, 1957–1962 (2003).
Perez-Juste, J., Liz-Marzán, L., Carnie, S., Chan, D. & Mulvaney, P. Electric-field-directed growth of gold nanorods in aqueous surfactant solutions. Adv. Funct. Mater. 14, 571–579 (2004).
Jana, N., Gearheart, L. & Murphy, C. Wet chemical synthesis of silver nanorods and nanowires of controllable aspect ratio. Chem. Commun. 617–618 (2001).
Prescott, S. & Mulvaney, P. Gold nanorod extinction spectra. J. Appl. Phys. 99,123504-1 (2006).
Sánchez-Iglesias, A. et al. Synthesis and optical properties of gold nanodecahedra with size control. Adv. Mater. 18, 2529–2534 (2006).
Gesquiere, A., Park, S. & Barbara, P. F-V/SMS: a new technique for studying the structure and dynamics of single molecules and nanoparticles. J. Phys. Chem. B 108, 10301–10308 (2004).
Acknowledgements
C.N. thanks the University of Melbourne for MIRS and MIFRS postgraduate scholarships and P.M. thanks the ARC for support through FF 0561456. We acknowledge I. Pastoriza-Santos for the gold pentagon samples.
Author information
Authors and Affiliations
Contributions
C.N. performed the experiments and analysed the data together with P.M. and A.M.F. A.M.F. provided help with the optics. All authors discussed the results and co-wrote the manuscript.
Corresponding author
Rights and permissions
About this article
Cite this article
Novo, C., Funston, A. & Mulvaney, P. Direct observation of chemical reactions on single gold nanocrystals using surface plasmon spectroscopy. Nature Nanotech 3, 598–602 (2008). https://doi.org/10.1038/nnano.2008.246
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2008.246
This article is cited by
-
Plasmon-mediated chemical reactions
Nature Reviews Methods Primers (2023)
-
Measuring and directing charge transfer in heterogenous catalysts
Nature Communications (2022)
-
Experimental characterization techniques for plasmon-assisted chemistry
Nature Reviews Chemistry (2022)
-
Revealing unconventional host–guest complexation at nanostructured interface by surface-enhanced Raman spectroscopy
Light: Science & Applications (2021)
-
In-situ nanospectroscopic imaging of plasmon-induced two-dimensional [4+4]-cycloaddition polymerization on Au(111)
Nature Communications (2021)