Abstract
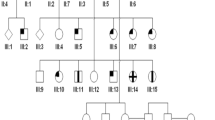
Inherited retinal dystrophies are the most common cause of childhood blindness in the developed world. Cone–rod retinal dystrophies are severe examples of this group of disorders. Analysis of a large cone–rod dystrophy pedigree suggested that inheritance within the family was influenced by meiotic drive (p=0.008), a rare segregation distortion in human genetics. Two–point linkage analysis showed significant linkage with three markers mapping to chromosome 19q. Multipoint analysis gave a maximum lod score of 10.08 (θ=0.05) distal to D19S47. Cone–rod dystrophy is therefore assigned to 19q13.1–q13.2 and a new candidate locus for other retinal dystrophies is identified.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Elston, J. Pediatric Ophthalmology (ed. D. Taylor) 3–6 (Blackwell Scientific Press, London, 1992).
Moore, A.T. Cone and cone-rod dystrophies. J. med. Genet. 29, 289–290 (1992).
Heckenlively, J.R., Martin, D.A. & Rosales, T.O. Telangectasia and optic atrophy in cone-rod degenerations. Arch. Ophthalmol. 99, 1983–1991 (1981).
Mantyjarvi, M. & Tuppurainen, K. Progressive cone-rod dystrophy and high myopia in a Finnish family. Arch. Ophthatmol. Copenh. 67, 234–242 (1989).
Heckenlively, J.R., Foxman, S.G. & Parelhoff, E.S. Retinal dystrophy and macular coloboma. Doc. Ophthalmol. 68, 257–271 (1988).
Bjork, A., Lindbau, U. & Wadensten, L. Retinal degenerations in hereditary ataxia. J. Neurol. Neurosurg. Psychiat. 19, 186–193 (1956).
Jalili, K. & Smith, N.J.D. A progressive cone-rod dystrophy and amelogenesis imperfecta: a new syndrome. J. med. Genet. 25, 738–740 (1988).
Samra, D., Abraham, F.A. & Treister, G. Inherited progressive cone-rod dystrophy and alopecia. Metab. Pediatr. Syst. Ophthalmol. 11, 83–85 (1988).
Kylstra, J.A. & Aylsworth, A.S. Cone-rod retinal dystrophy in a patient with neurofibromatosis type 1. Can. J. Ophthalmol. 28, 79–80 (1993).
Jalili, I.K. Cone-rod congenital amaurosis associated with congenital hypertrichosis: an autosomai recessive condition. J. med. Genet. 26, 504–510 (1989).
Berson, E.L., Gouras, P. & Gunkel, R.D. Progressive cone-rod degenerations. Arch. Ophthalmol. 80, 68–76 (1968).
Al-Maghtheh, M., Gregory, C., Inglehearn, C., Hardcastle, A. & Bhattacharya, S. Rhodopsin mutations in autosomal dominant retinitis pigmentosa. Hum. Mut. 2, 249–255 (1993).
Wells, J. et al. Mutations in human retinal degeneration slow (RDS) gene can cause either retinitis pigmentosa or macular dystrophy. Nature Genet. 3, 213–218 (1993).
Stone, E.D., Nichols, B.E., Streb, L.M., Kimura, A.E. & Sheffield, V.C. Genetic linkage of vitelliform macular degeneration (Best's disease) to chromosome 11q13. Nature Genet. 1, 246–250 (1992).
Ferrell, R.E., Hittner, H.M. & Chakravarti, A. Autosomal dominant cone-rod dystrophy: a linkage study with 17 biochemical and serological markers. Am. J. med. Genet. 8, 363–369 (1981).
Warburg, M., Sjö, O. & Fledelius, H.C. Deletion mapping of a retinal cone-rod dystrophy: assignment to 18q211. Am. J. med. Genet. 39, 288–293 (1991).
Yagasaki, K. & Jacobson, S.G. Cone-rod dystrophy. Phenotypic diversity by retinal function testing. Arch. Ophthalmol. 107, 701–708 (1989).
Weissenbach, J. et al. A second generation linkage map of the human genome. Nature 359, 794–801 (1992).
NIH/CEPH Collaborative mapping group. A comprehensive genetic linkage map of the human genome. Science 258, 67–86 (1992).
Krill, A.E., Deutman, A.F. & Fishman, M. The cone degenerations. Doc. Ophthalmol. 35, 1–80 (1973).
Jansen, G. et al. Physical and genetic characterization of the distal segment of the myotonic dystrophy area on 19q. Genomics 13, 509–517 (1992).
Lang, G.E. & Maumenee, I.H. Retinal dystrophies associated with storage diseases. Retinal dystrophies end degenerations (ed. D.A. Newsome) 331–333 (Raven Press, New York, 1988).
Burian, H.M. & Burns, C.A. Ocular changes in myotonic dystrophy. Am. J. Ophthalmol. 63, 22–34 (1967).
Travis, G.H. & Hepler, J.E. A medley of retinal dystrophies. Nature Genet. 3, 191–192 (1993).
Gass, J.D.M., Atlas of Macular Diseases 60–91 (C.V. Mosby, St. Louis, 1987).
Magenis, R.E. et al. Comparison of the 15q deletions in Prader-Willi and Angelman syndromes: specific regions, extent of deletions, parental origin, and clinical consequences. Am. J. med. Genet. 35, 333–349 (1990).
Richards, R.I. et al. Evidence of founder chromosomes in fragile X syndrome. Nature Genet. 1, 257–260 (1992).
Lyttle, T.W. Segregation Distorters. A. Rev. Genet. 25, 511–557 (1991).
Hiraizumi, Y. & Martin, D.W. On the models of segregation distortion in Drosophila melanogaster. Genetics 93, 423–435 (1979).
Bennett, D. & Dunn, L.C. Transmission ratio distorting genes on chromosome IX and their interactions. Proc. Symp. Immunogenetics of the H-2 system. (eds Lengerova, A. & Vojtiskova, M.) 90–103 (Karger, Basel, 1971).
Small, K.W. Genetic segregation distortion in MCDR1 (North Carolina macular dystrophy). Invest. Ophthalmol. Vis. Sci. (ARVO abstracts) 34, 1305 (1993).
Saiki, R.K. et al. Primer-directed enzymic amplification of DNA with a thermostable DNA polymerase. Science 239, 487–491 (1988).
Attwood, J. & Bryant, S. A computer programme to make analysis with Liped and Linkage easier to perform and less prone to input errors. A. hum. Genet. 52, 259 (1988).
Lathrop, G.M., Lalouel, J.M., Julier, C. & Ott, J. Strategies for multipoint linkage analysis in humans. Proc. natn. Acad. Sci. U.S.A. 81, 3443–3446 (1984).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Evans, K., Fryer, A., Inglehearn, C. et al. Genetic linkage of cone–rod retinal dystrophy to chromosome 19q and evidence for segregation distortion. Nat Genet 6, 210–213 (1994). https://doi.org/10.1038/ng0294-210
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ng0294-210
This article is cited by
-
Clinical and Genetic Characteristics of 18 Patients from 13 Japanese Families with CRX-associated retinal disorder: Identification of Genotype-phenotype Association
Scientific Reports (2020)
-
LRRTM4-C538Y novel gene mutation is associated with hereditary macular degeneration with novel dysfunction of ON-type bipolar cells
Journal of Human Genetics (2018)
-
A Note on the Power to Detect Transmission Distortion in Parent-Child Trios via the Transmission Disequilibrium Test
Behavior Genetics (2006)
-
Refinement of the locus for autosomal recessive cone–rod dystrophy (CORD8) linked to chromosome 1q23–q24 in a Pakistani family and exclusion of candidate genes
Journal of Human Genetics (2006)
-
Transmission ratio distortion at the INS-IGF2 VNTR
Nature Genetics (1999)