Abstract
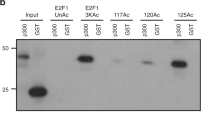
E2F1, a member of the E2F family of transcription factors, in addition to its established proliferative effect1, has also been implicated in the induction of apoptosis through p53-dependent and p53-independent pathways2. Several genes involved in the activation or execution of the apoptotic programme have recently been shown to be upregulated at the transcriptional level by E2F1 overexpression, including the genes encoding INK4a/ARF, Apaf-1, caspase 7 and p73 (refs 3–5). E2F1 is stabilized in response to DNA damage6,7 but it has not been established how this translates into the activation of specific subsets of E2F target genes. Here, we applied a chromatin immunoprecipitation approach to show that, in response to DNA damage, E2F1 is directed from cell cycle progression to apoptotic E2F target genes. We identify p73 as an important E2F1 apoptotic target gene in DNA damage response and we show that acetylation is required for E2F1 recruitment on the P1p73 promoter and for its transcriptional activation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Muller, H. & Helin, K. The E2F transcription factors: key regulators of cell proliferation. Biochim. Biophys. Acta 1470, M1–M12 (2000).
Phillips, A.C. & Vousden, K.H. E2F-1 induced apoptosis. Apoptosis 6, 173–182 (2001).
Muller, H. et al. E2Fs regulate the expression of genes involved in differentiation, development, proliferation, and apoptosis. Genes Dev. 15, 267–285 (2001).
Stanelle, J., Stiewe, T., Theseling, C.C., Peter, M. & Putzer, B.M. Gene expression changes in response to E2F1 activation. Nucleic Acids Res. 30, 1859–1867 (2002).
Nahle, Z. et al. Direct coupling of the cell cycle and cell death machinery by E2F. Nature Cell Biol. 4, 859–864 (2002).
Blattner, C., Sparks, A. & Lane, D. Transcription factor E2F-1 is upregulated in response to DNA damage in a manner analogous to that of p53. Mol. Cell. Biol. 19, 3704–3713 (1999).
Lin, W.C., Lin, F.T. & Nevins, J.R. Selective induction of E2F1 in response to DNA damage, mediated by ATM-dependent phosphorylation. Genes Dev. 15, 1833–1844 (2001).
Ishida, S. et al. Role for E2F in control of both DNA replication and mitotic functions as revealed from DNA microarray analysis. Mol. Cell. Biol. 21, 4684–4699 (2001).
Ren, B. et al. E2F integrates cell cycle progression with DNA repair, replication, and G2/M checkpoints. Genes Dev. 16, 245–256 (2002).
Wells, J., Graveel, C.R., Bartley, S.M., Madore, S.J. & Farnham, P.J. The identification of E2F1-specific target genes. Proc. Natl Acad. Sci. USA 99, 3890–3895 (2002).
Bates, S. et al. p14ARF links the tumour suppressors RB and p53. Nature 395, 124–125 (1998).
Moroni, M.C. et al. Apaf-1 is a transcriptional target for E2F and p53. Nature Cell Biol. 3, 552–558 (2001).
Lissy, N.A., Davis, P.K., Irwin, M., Kaelin, W.G. & Dowdy, S.F. A common E2F-1 and p73 pathway mediates cell death induced by TCR activation. Nature 407, 642–645 (2000).
Agami, R., Blandino, G., Oren, M. & Shaul, Y. Interaction of c-Abl and p73α and their collaboration to induce apoptosis. Nature 399, 809–813 (1999).
Costanzo, A. et al. DNA damage-dependent acetylation of p73 dictates the selective activation of apoptotic target genes. Mol. Cell 9, 175–186 (2002).
Flores, E.R. et al. p63 and p73 are required for p53-dependent apoptosis in response to DNA damage. Nature 416, 560–564 (2002).
Gong, J.G. et al. The tyrosine kinase c-Abl regulates p73 in apoptotic response to cisplatin-induced DNA damage. Nature 399, 806–809 (1999).
Irwin, M. et al. Role for the p53 homologue p73 in E2F-1-induced apoptosis. Nature 407, 645–648 (2000).
Stiewe, T. & Putzer, B.M. Role of the p53-homologue p73 in E2F1-induced apoptosis. Nature Genet. 26, 464–469 (2000).
Vossio, S. et al. DN-p73 is activated after DNA damage in a p53-dependent manner to regulate p53-induced cell cycle arrest. Oncogene 21, 3796–3803 (2002).
Takahashi, Y., Rayman, J.B. & Dynlacht, B.D. Analysis of promoter binding by the E2F and pRB families in vivo: Distinct E2F proteins mediate activation and repression. Genes Dev. 14, 804–816 (2000).
Luo, R.X., Postigo, A.A. & Dean, D.C. Rb interacts with histone deacetylase to repress transcription. Cell 92, 463–473 (1998).
Martinez-Balbas, M.A., Bauer, U.M., Nielsen, S.J., Brehm, A. & Kouzarides, T. Regulation of E2F1 activity by acetylation. EMBO J. 19, 662–671 (2000).
Zhang, Q., Yao, H., Vo, N. & Goodman, R.H. Acetylation of adenovirus E1A regulates binding of the transcriptional corepressor CtBP. Proc. Natl Acad. Sci. USA 97, 14323–14328 (2000).
Chan, H.M., Krstic-Demonacos, M., Smith, L., Demonacos, C. & La Thangue, N.B. Acetylation control of the retinoblastoma tumour-suppressor protein. Nature Cell Biol. 3, 667–674 (2001).
Chen, L.F., Mu, Y. & Green, W.C. Acetylation of RelA at discrete sites regulates distinct nuclear functions of NF-κB. EMBO J. 21, 6539–6548 (2002).
Sartorelli, V. et al. Acetylation of MyoD directed by PCAF is necessary for the execution of the muscle program. Mol. Cell 4, 725–734 (1999).
Trouche, D. & Kouzarides, T. E2F1 and E1A(12S) have a homologous activation domain regulated by RB and CBP. Proc. Natl Acad. Sci. USA 93, 1439–1442 (1996).
Caplen, N.J., Parrish, S., Imani, F., Fire, A. & Morgan, R.A. Specific inhibition of gene expression by small double-stranded RNAs in invertebrate and vertebrate systems. Proc. Natl Acad. Sci. USA 98, 9742–9747 (2001).
Acknowledgements
We thank P. Lavia, G. Blandino, P.L. Puri, M. Fanciulli and E. De Smaele for reading the manuscript critically and for helpful suggestions. This work was supported by grants from AIRC, MURST-Cofin, MURST-FIRB, Ministry of Health and Telethon to M.L., from AIRC, the Ministry of University and Research, National Research Council – 'Oncology' Project, the Ministry of Health MURST-CNR 'Biomolecole per la Salute Umana' Program and Center of Excellence BEMM to A.G.; from AIRC and Cenci-Bolognetti Foundation, Pasteur Institute, to I.S. and from the Ministry of Health to E.A. N.P. and L.B. are supported by fellowships from the Fondazione A. Cesalpino. R.G. is supported by a fellowship from FIRC.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information Fig. S1
Supplementary Information Fig. S2 (PDF 42 kb)
Supplementary Information Fig. S3
Supplementary Information Fig. S4
Rights and permissions
About this article
Cite this article
Pediconi, N., Ianari, A., Costanzo, A. et al. Differential regulation of E2F1 apoptotic target genes in response to DNA damage. Nat Cell Biol 5, 552–558 (2003). https://doi.org/10.1038/ncb998
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb998
This article is cited by
-
Context-Dependent Functions of E2F1: Cell Cycle, Cell Death, and DNA Damage Repair in Cortical Neurons
Molecular Neurobiology (2020)
-
Programmed expression of pro-apoptotic BMCC1 during apoptosis, triggered by DNA damage in neuroblastoma cells
BMC Cancer (2019)
-
E2F1 acetylation directs p300/CBP-mediated histone acetylation at DNA double-strand breaks to facilitate repair
Nature Communications (2019)
-
Biological processes and signal transduction pathways regulated by the protein methyltransferase SETD7 and their significance in cancer
Signal Transduction and Targeted Therapy (2018)
-
DNA damage and S phase-dependent E2F1 stabilization requires the cIAP1 E3-ubiquitin ligase and is associated with K63-poly-ubiquitination on lysine 161/164 residues
Cell Death & Disease (2017)