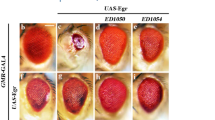
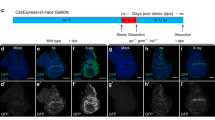
Abstract
Proliferation and apoptosis must be precisely regulated to form organs with appropriate cell numbers and to avoid tumour growth1,2. Here we show that Hippo (Hpo), the Drosophila homologue of the mammalian Ste20-like kinases3, MST1/2, promotes proper termination of cell proliferation and stimulates apoptosis during development. hpo mutant tissues are larger than normal because mutant cells continue to proliferate beyond normal tissue size and are resistant to apoptotic stimuli that usually eliminate extra cells. Hpo negatively regulates expression of Cyclin E to restrict cell proliferation, downregulates the Drosophila inhibitor of apoptosis protein DIAP1, and induces the proapoptotic gene head involution defective (hid) to promote apoptosis. The mutant phenotypes of hpo are similar to those of warts (wts), which encodes a serine/threonine kinase of the myotonic dystrophy protein kinase family4,5, and salvador (sav), which encodes a WW domain protein that binds to Wts6,7. We find that Sav binds to a regulatory domain of Hpo that is essential for its function, indicating that Hpo acts together with Sav and Wts in a signalling module that coordinately regulates cell proliferation and apoptosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Conlon, I. & Raff, M. Size control in animal development. Cell 96, 235–244 (1999).
Green, D.R. & Evan, G.I. A matter of life and death. Cancer Cell 1, 19–30 (2002).
Dan, I., Watanabe, N.M. & Kusumi, A. The Ste20 group kinases as regulators of MAP kinase cascades. Trends Cell Biol. 11, 220–230 (2001).
Justice, R.W., Zilian, O., Woods, D.F., Noll, M. & Bryant, P.J. The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev. 9, 534–546 (1995).
Xu, T., Wang, W., Zhang, S., Stewart, R.A. & Yu, W. Identifying tumor suppressors in genetic mosaics: the Drosophila lats gene encodes a putative protein kinase. Development 121, 1053–1063 (1995).
Kango-Singh, M. et al. Shar-pei mediates cell proliferation arrest during imaginal disc growth in Drosophila. Development 129, 5719–5730 (2002).
Tapon, N. et al. salvador promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines. Cell 110, 468–478 (2002).
Newsome, T.P., Asling, B. & Dickson, B.J. Analysis of Drosophila photoreceptor axon guidance in eye-specific mosaics. Development 127, 851–860 (2000).
Baker, N.E. Cell proliferation, survival, and death in the Drosophila eye. Semin. Cell Dev. Biol. 12, 499–507 (2001).
Neufeld, T.P., de la Cruz, A.F., Johnston, L.A. & Edgar, B.A. Coordination of growth and cell division in the Drosophila wing. Cell 93, 1183–1193 (1998).
Grether, M.E., Abrams, J.M., Agapite, J., White, K. & Steller, H. The head involution defective gene of Drosophila melanogaster functions in programmed cell death. Genes Dev. 9, 1694–1708 (1995).
White, K., Tahaoglu, E. & Steller, H. Cell killing by the Drosophila gene reaper. Science 271, 805–807 (1996).
Chen, P., Nordstrom, W., Gish, B. & Abrams, J.M. grim, a novel cell death gene in Drosophila. Genes Dev. 10, 1773–1782 (1996).
Christich, A. et al. The damage-responsive Drosophila gene sickle encodes a novel IAP binding protein similar to but distinct from reaper, grim, and hid. Curr. Biol. 12, 137–140 (2002).
Wing, J.P. et al. Drosophila sickle is a novel grim-reaper cell death activator. Curr. Biol. 12, 131–135 (2002).
Srinivasula, S.M. et al. sickle, a novel Drosophila death gene in the reaper/hid/grim region, encodes an IAP-inhibitory protein. Curr. Biol. 12, 125–130 (2002).
Tenev, T., Zachariou, A., Wilson, R., Paul, A. & Meier, P. Jafrac2 is an IAP antagonist that promotes cell death by liberating Dronc from DIAP1. EMBO J. 21, 5118–5129 (2002).
Yoo, S.J. et al. Hid, Rpr and Grim negatively regulate DIAP1 levels through distinct mechanisms. Nature Cell Biol. 4, 416–424 (2002).
Wang, S.L., Hawkins, C.J., Yoo, S.J., Muller, H.A. & Hay, B.A. The Drosophila caspase inhibitor DIAP1 is essential for cell survival and is negatively regulated by HID. Cell 98, 453–463 (1999).
Lisi, S., Mazzon, I. & White, K. Diverse domains of THREAD/DIAP1 are required to inhibit apoptosis induced by REAPER and HID in Drosophila. Genetics 154, 669–678 (2000).
Goyal, L., McCall, K., Agapite, J., Hartwieg, E. & Steller, H. Induction of apoptosis by Drosophila reaper, hid and grim through inhibition of IAP function. EMBO J. 19, 589–597 (2000).
Berger, J. et al. Genetic mapping with SNP markers in Drosophila. Nature Genet. 29, 475–481 (2001).
Cheung, W.L. et al. Apoptotic phosphorylation of histone H2B is mediated by mammalian sterile twenty kinase. Cell 113, 507–517 (2003).
Graves, J.D. et al. Caspase-mediated activation and induction of apoptosis by the mammalian Ste20-like kinase Mst1. EMBO J. 17, 2224–2234 (1998).
Lee, K.K., Ohyama, T., Yajima, N., Tsubuki, S. & Yonehara, S. MST, a physiological caspase substrate, highly sensitizes apoptosis both upstream and downstream of caspase activation. J. Biol. Chem. 276, 19276–19285 (2001).
Creasy, C.L., Ambrose, D.M. & Chernoff, J. The Ste20-like protein kinase, Mst1, dimerizes and contains an inhibitory domain. J. Biol. Chem. 271, 21049–21053 (1996).
Kurada, P. & White, K. Ras promotes cell survival in Drosophila by downregulating hid expression. Cell 95, 319–329 (1998).
Bergmann, A., Agapite, J., McCall, K. & Steller, H. The Drosophila gene hid is a direct molecular target of Ras-dependent survival signaling. Cell 95, 331–341 (1998).
Yang, L. & Baker, N.E. Cell cycle withdrawal, progression, and cell survival regulation by EGFR and its effectors in the differentiating Drosophila eye. Dev. Cell 4, 359–369 (2003).
Deng, Y., Pang, A. & Wang, J.H. Regulation of mammalian STE20-like kinase 2 (MST2) by protein phosphorylation/dephosphorylation and proteolysis. J. Biol. Chem. 278, 11760–11767 (2003).
Johnston, L.A. & Gallant, P. Control of growth and organ size in Drosophila. BioEssays 24, 54–64 (2002).
Oldham, S. & Hafen, E. Insulin/IGF and target of rapamycin signaling: a TOR de force in growth control. Trends Cell Biol. 13, 79–85 (2003).
Turenchalk, G.S., St John, M.A., Tao, W. & Xu, T. The role of LATS in cell cycle regulation and tumorigenesis. Biochim. Biophys. Acta 1424, M9–M16 (1999).
Brand, A.H. & Perrimon, N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401–415 (1993).
Acknowledgements
We thank K. Basler, H. J. Bellen, A. Bergmann, P. Bryant, K.-W. Choi, B. Dickson, B. Edgar, B. Hay, G. Mardon, M. Miura, A. Singh, the Bloomington Drosophila Stock Center, and the Developmental Studies Hybridoma Bank for fly stocks and antibodies; H. Jafar-Nejad for technical advice with the yeast-two-hybrid screen; G. Zhai for help with SNP detection by HPLC; P. R. Hiesinger for help with pupal stainings; J. Zhang for help with microinjections; K. Dunner for help with SEM, which along with DNA sequencing was done at M. D. Anderson core facilities, which is supported by a grant from the National Cancer Institute (CA16672); L. McCord for help with artwork; and H. J. Bellen, A. Bergmann, B. Frankfort, P. R. Hiesinger, R. Johnson, J. Kunz, S. Markus, K. Pappu, A. Singh and G. Zhai for discussion and comments on the manuscript. This publication was made possible by grants from the NIEHS (T32 ES07332) and the NICHD (HD07325) to R.U., and an NIH grant (GM067997), a Pharmacia Research Grant and a Basil O'Connor Award (FY01-497) to G.H.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information, Fig.S1
Supplementary Information, Fig.S2 (PDF 394 kb)
Supplementary Information, Fig.S3
Supplementary Information, Fig.S4
Supplementary Information, Fig.S5
Supplementary Information, Fig.S6
Rights and permissions
About this article
Cite this article
Udan, R., Kango-Singh, M., Nolo, R. et al. Hippo promotes proliferation arrest and apoptosis in the Salvador/Warts pathway. Nat Cell Biol 5, 914–920 (2003). https://doi.org/10.1038/ncb1050
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1050
This article is cited by
-
miR-277 targets the proapoptotic gene-hid to ameliorate Aβ42-mediated neurodegeneration in Alzheimer’s model
Cell Death & Disease (2024)
-
Maintenance of appropriate size scaling of the C. elegans pharynx by YAP-1
Nature Communications (2023)
-
Context-dependent transcriptional regulations of YAP/TAZ in stem cell and differentiation
Stem Cell Research & Therapy (2022)
-
Mechanical regulation of bone remodeling
Bone Research (2022)
-
Reduced expression of TAZ inhibits primary cilium formation in renal glomeruli
Experimental & Molecular Medicine (2022)