Abstract
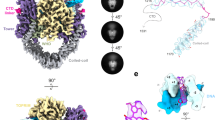
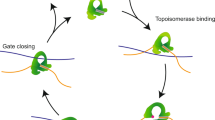
Type II topoisomerases disentangle DNA to facilitate chromosome segregation, and represent a major class of therapeutic targets. Although these enzymes have been studied extensively, a molecular understanding of DNA binding has been lacking. Here we present the structure of a complex between the DNA-binding and cleavage core of Saccharomyces cerevisiae Topo II (also known as Top2) and a gate-DNA segment. The structure reveals that the enzyme enforces a 150° DNA bend through a mechanism similar to that of remodelling proteins such as integration host factor. Large protein conformational changes accompany DNA deformation, creating a bipartite catalytic site that positions the DNA backbone near a reactive tyrosine and a coordinated magnesium ion. This configuration closely resembles the catalytic site of type IA topoisomerases, reinforcing an evolutionary link between these structurally and functionally distinct enzymes. Binding of DNA facilitates opening of an enzyme dimerization interface, providing visual evidence for a key step in DNA transport.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Wang, J. C. Cellular roles of DNA topoisomerases: a molecular perspective. Nature Rev. Mol. Cell Biol. 3, 430–440 (2002)
Gadelle, D., Filee, J., Buhler, C. & Forterre, P. Phylogenomics of type II DNA topoisomerases. Bioessays 25, 232–242 (2003)
Hande, K. R. Clinical applications of anticancer drugs targeted to topoisomerase II. Biochim. Biophys. Acta 1400, 173–184 (1998)
Hooper, D. C. & Rubinstein, E. Quinolone antimicrobial agents xiv (ASM, Washington DC, 2003)
Roca, J., Berger, J. M., Harrison, S. C. & Wang, J. C. DNA transport by a type II topoisomerase: direct evidence for a two-gate mechanism. Proc. Natl Acad. Sci. USA 93, 4057–4062 (1996)
Roca, J. & Wang, J. C. The capture of a DNA double helix by an ATP-dependent protein clamp: a key step in DNA transport by type II DNA topoisomerases. Cell 71, 833–840 (1992)
Roca, J. & Wang, J. C. DNA transport by a type II DNA topoisomerase: evidence in favor of a two-gate mechanism. Cell 77, 609–616 (1994)
Williams, N. L. & Maxwell, A. Probing the two-gate mechanism of DNA gyrase using cysteine cross-linking. Biochemistry 38, 13502–13511 (1999)
Berger, J. M., Gamblin, S. J., Harrison, S. C. & Wang, J. C. Structure and mechanism of DNA topoisomerase II. Nature 379, 225–232 (1996)
Aravind, L., Leipe, D. D. & Koonin, E. V. Toprim—a conserved catalytic domain in type IA and II topoisomerases, DnaG-type primases, OLD family nucleases and RecR proteins. Nucleic Acids Res. 26, 4205–4213 (1998)
Noble, C. G. & Maxwell, A. The role of GyrB in the DNA cleavage–religation reaction of DNA gyrase: a proposed two metal-ion mechanism. J. Mol. Biol. 318, 361–371 (2002)
Osheroff, N. Role of the divalent cation in topoisomerase II mediated reactions. Biochemistry 26, 6402–6406 (1987)
Goto, T., Laipis, P. & Wang, J. C. The purification and characterization of DNA topoisomerases I and II of the yeast Saccharomyces cerevisiae. J. Biol. Chem. 259, 10422–10429 (1984)
Lima, C. D., Wang, J. C. & Mondragon, A. Three-dimensional structure of the 67K N-terminal fragment of E. coli DNA topoisomerase I. Nature 367, 138–146 (1994)
Berger, J. M., Fass, D., Wang, J. C. & Harrison, S. C. Structural similarities between topoisomerases that cleave one or both DNA strands. Proc. Natl Acad. Sci. USA 95, 7876–7881 (1998)
Podobnik, M., McInerney, P., O’Donnell, M. & Kuriyan, J. A. TOPRIM domain in the crystal structure of the catalytic core of Escherichia coli primase confirms a structural link to DNA topoisomerases. J. Mol. Biol. 300, 353–362 (2000)
Keck, J. L., Roche, D. D., Lynch, A. S. & Berger, J. M. Structure of the RNA polymerase domain of E. coli primase. Science 287, 2482–2486 (2000)
Howard, M. T., Lee, M. P., Hsieh, T. S. & Griffith, J. D. Drosophila topoisomerase II–DNA interactions are affected by DNA structure. J. Mol. Biol. 217, 53–62 (1991)
Zechiedrich, E. L. & Osheroff, N. Eukaryotic topoisomerases recognize nucleic acid topology by preferentially interacting with DNA crossovers. EMBO J. 9, 4555–4562 (1990)
Roca, J., Berger, J. M. & Wang, J. C. On the simultaneous binding of eukaryotic DNA topoisomerase II to a pair of double-stranded DNA helices. J. Biol. Chem. 268, 14250–14255 (1993)
Vologodskii, A. V. et al. Mechanism of topology simplification by type II DNA topoisomerases. Proc. Natl Acad. Sci. USA 98, 3045–3049 (2001)
Berger, J. M. Structural determination of a 92 kDa fragment of yeast topoisomerase II by X-ray crystallography at 2.7 Å resolution. PhD thesis, Harvard University. (1995)
Fass, D., Bogden, C. E. & Berger, J. M. Quaternary changes in topoisomerase II may direct orthogonal movement of two DNA strands. Nature Struct. Biol. 6, 322–326 (1999)
Thomsen, B. et al. Characterization of the interaction between topoisomerase II and DNA by transcriptional footprinting. J. Mol. Biol. 215, 237–244 (1990)
Orphanides, G. & Maxwell, A. Evidence for a conformational change in the DNA gyrase–DNA complex from hydroxyl radical footprinting. Nucleic Acids Res. 22, 1567–1575 (1994)
Wang, Y., Thyssen, A., Westergaard, O. & Andersen, A. H. Position-specific effect of ribonucleotides on the cleavage activity of human topoisomerase II. Nucleic Acids Res. 28, 4815–4821 (2000)
Liu, Q. & Wang, J. C. Identification of active site residues in the “GyrA” half of yeast DNA topoisomerase II. J. Biol. Chem. 273, 20252–20260 (1998)
Rice, P. A., Yang, S., Mizuuchi, K. & Nash, H. A. Crystal structure of an IHF–DNA complex: a protein-induced DNA U-turn. Cell 87, 1295–1306 (1996)
Lu, X. J. & Olson, W. K. 3DNA: a software package for the analysis, rebuilding and visualization of three-dimensional nucleic acid structures. Nucleic Acids Res. 31, 5108–5121 (2003)
Morais Cabral, J. H. et al. Crystal structure of the breakage–reunion domain of DNA gyrase. Nature 388, 903–906 (1997)
Tse, Y. C., Kirkegaard, K. & Wang, J. C. Covalent bonds between protein and DNA. Formation of phosphotyrosine linkage between certain DNA topoisomerases and DNA. J. Biol. Chem. 255, 5560–5565 (1980)
Domanico, P. L. & Tse-Dinh, Y. C. Mechanistic studies on E. coli DNA topoisomerase I: divalent ion effects. J. Inorg. Biochem. 42, 87–96 (1991)
Sugino, A., Higgins, N. P., Brown, P. O., Peebles, C. L. & Cozzarelli, N. R. Energy coupling in DNA gyrase and the mechanism of action of novobiocin. Proc. Natl Acad. Sci. USA 75, 4838–4842 (1978)
Osheroff, N. Eukaryotic topoisomerase II. Characterization of enzyme turnover. J. Biol. Chem. 261, 9944–9950 (1986)
Trigueros, S., Salceda, J., Bermudez, I., Fernandez, X. & Roca, J. Asymmetric removal of supercoils suggests how topoisomerase II simplifies DNA topology. J. Mol. Biol. 335, 723–731 (2004)
Rybenkov, V. V., Ullsperger, C., Vologodskii, A. V. & Cozzarelli, N. R. Simplification of DNA topology below equilibrium values by type II topoisomerases. Science 277, 690–693 (1997)
Buck, G. R. & Zechiedrich, E. L. DNA disentangling by type-2 topoisomerases. J. Mol. Biol. 340, 933–939 (2004)
Baker, N. A., Sept, D., Joseph, S., Holst, M. J. & McCammon, J. A. Electrostatics of nanosystems: application to microtubules and the ribosome. Proc. Natl Acad. Sci. USA 98, 10037–10041 (2001)
DeLano, W. The PyMOL molecular graphics system. 〈http://www.pymol.org〉 (2002)
Worland, S. T. & Wang, J. C. Inducible overexpression, purification, and active site mapping of DNA topoisomerase II from the yeast Saccharomyces cerevisiae. J. Biol. Chem. 264, 4412–4416 (1989)
Kapust, R. B. & Waugh, D. S. Escherichia coli maltose-binding protein is uncommonly effective at promoting the solubility of polypeptides to which it is fused. Protein Sci. 8, 1668–1674 (1999)
Davies, D. R. & Hol, W. G. The power of vanadate in crystallographic investigations of phosphoryl transfer enzymes. FEBS Lett. 577, 315–321 (2004)
MacDowell, A. A. et al. Suite of three protein crystallography beamlines with single superconducting bend magnet as the source. J. Synchrotron Radiat. 11, 447–455 (2004)
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997)
McCoy, A. J., Grosse-Kunstleve, R. W., Storoni, L. C. & Read, R. J. Likelihood-enhanced fast translation functions. Acta Crystallogr. D 61, 458–464 (2005)
Potterton, E., Briggs, P., Turkenburg, M. & Dodson, E. A graphical user interface to the CCP4 program suite. Acta Crystallogr. D 59, 1131–1137 (2003)
Changela, A., DiGate, R. J. & Mondragon, A. Crystal structure of a complex of a type IA DNA topoisomerase with a single-stranded DNA molecule. Nature 411, 1077–1081 (2001)
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004)
Adams, P. D. et al. PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr. D 58, 1948–1954 (2002)
Davis, I. W. et al. MolProbity: all-atom contacts and structure validation for proteins and nucleic acids. Nucleic Acids Res. 35, W375–W383 (2007)
Acknowledgements
The authors are grateful to D. Herschlag for advice on the choice of DNA substrate, and to members of the Berger laboratory for discussions. This work was supported by a NIH Training Grant position to K.C.D. and by the NCI.
Author Contributions K.C.D. and J.M.B. designed the experimental plan, analysed the data, and wrote the paper. K.C.D. performed the research.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information
The file contains Supplementary Table 1 and Supplementary Figures S1-S9 with Legends, Legend to Supplementary Movie 1 and additional references. (PDF 12278 kb)
Supplementary Movie 1
The file contains Supplementary Movie 1. (MOV 26939 kb)
Rights and permissions
About this article
Cite this article
Dong, K., Berger, J. Structural basis for gate-DNA recognition and bending by type IIA topoisomerases. Nature 450, 1201–1205 (2007). https://doi.org/10.1038/nature06396
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature06396
This article is cited by
-
Etoposide promotes DNA loop trapping and barrier formation by topoisomerase II
Nature Chemical Biology (2023)
-
Chromatinization modulates topoisomerase II processivity
Nature Communications (2023)
-
Evolutionary History of TOPIIA Topoisomerases in Animals
Journal of Molecular Evolution (2022)
-
The pivot point arginines identified in the β-pinwheel structure of C-terminal domain from Salmonella Typhi DNA Gyrase A subunit
Scientific Reports (2020)
-
Topoisomerase IIβ targets DNA crossovers formed between distant homologous sites to induce chromatin opening
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.