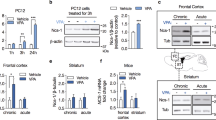
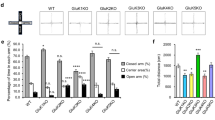
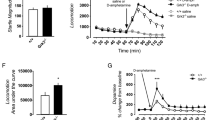
Abstract
The glutamate receptor 6 (GluR6 or GRIK2, one of the kainate receptors) gene resides in a genetic linkage region (6q21) associated with bipolar disorder (BPD), but its function in affective regulation is unknown. Compared with wild-type (WT) and GluR5 knockout (KO) mice, GluR6 KO mice were more active in multiple tests and super responsive to amphetamine. In a battery of specific tests, GluR6 KO mice also exhibited less anxious or more risk-taking type behavior and less despair-type manifestations, and they also had more aggressive displays. Chronic treatment with lithium, a classic antimanic mood stabilizer, reduced hyperactivity, aggressive displays and some risk-taking type behavior in GluR6 KO mice. Hippocampal and prefrontal cortical membrane levels of GluR5 and KA-2 receptors were decreased in GluR6 KO mice, and chronic lithium treatment did not affect these decreases. The membrane levels of other glutamatergic receptors were not significantly altered by GluR6 ablation or chronic lithium treatment. Together, these biochemical and behavioral results suggest a unique role for GluR6 in controlling abnormalities related to the behavioral symptoms of mania, such as hyperactivity or psychomotor agitation, aggressiveness, driven or increased goal-directed pursuits, risk taking and supersensitivity to psychostimulants. Whether GluR6 perturbation is involved in the mood elevation or thought disturbance of mania and the cyclicity of BPD are unknown. The molecular mechanism underlying the behavioral effects of lithium in GluR6 KO mice remains to be elucidated.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout














Similar content being viewed by others
References
Goodwin FK, Jamison KR . Manic-Depressive Illness: Bipolar Disorders and Recurrent Depression. Oxford University Press: New York, 2007.
Baum A, Akula N, Cabenero M, Cardona I, Corona W, Klemens B et al. A genome-wide association study implicates diacylglycerol kinase eta (DGKH) and several other genes in the etiology of bipolar disorder. Mol Psychiatry 2007; 13: 197–207.
Kato T . Molecular genetics of bipolar disorder and depression. Psychiatry Clin Neurosci 2007; 61: 3–19.
The Wellcome Trust Case Control Consortium. Genome-wide association study of 14 000 cases of seven common diseases and 3000 shared controls. Nature 2007; 447: 661–678.
Buervenich S, Detera-Wadleigh SD, Akula N, Thomas CJM, Kassem L, Rezvani A et al. Fine mapping on chromosome 6q in the NIMH Genetics Initiative bipolar pedigrees (abstract 1882). Annual Meeting of The American Society of Human Genetics. Los Angeles, CA, 2003 (http://www.genetics.faseb.org/genetics/ashg03s/index.shtml).
Dick DM, Foroud T, Flury L, Bowman ES, Miller MJ, Rau NL et al. Genomewide linkage analyses of bipolar disorder: a new sample of 250 pedigrees from the National Institute of Mental Health Genetics Initiative. Am J Hum Genet 2003; 73: 107–114.
McQueen MB, Devlin B, Faraone SV, Nimgaonkar VL, Sklar P, Smoller JW et al. Combined analysis from eleven linkage studies of bipolar disorder provides strong evidence of susceptibility loci on chromosomes 6q and 8q. Am J Hum Genet 2005; 77: 582–595.
Schulze TG, Buervenich S, Badner JA, Steele CJ, Detera-Wadleigh SD, Dick DM et al. Loci on chromosomes 6q and 6p interact to increase susceptibility to bipolar affective disorder in the national institute of mental health genetics initiative pedigrees. Biol Psychiatry 2004; 56: 18–23.
Schumacher J, Kaneva R, Jamra RA, Diaz GO, Ohlraun S, Milanova V et al. Genomewide scan and fine-mapping linkage studies in four European samples with bipolar affective disorder suggest a new susceptibility locus on chromosome 1p35–p36 and provides further evidence of loci on chromosome 4q31 and 6q24. Am J Hum Genet 2005; 77: 1102–1111.
Beneyto M, Kristiansen LV, Oni-Orisan A, McCullumsmith RE, Meador-Woodruff JH . Abnormal glutamate receptor expression in the medial temporal lobe in schizophrenia and mood disorders. Neuropsychopharmacology 2007; 14: 14.
Laje G, Buervenich S, Manji HK, Rush AJ, Wilson A, Charney DS et al. Genetic markers of suicidal ideation emerging during citalopram treatment of major depression. Am J Psychiatry 2007; 164: 1530–1538.
McElroy SL, Kotwal R, Kaneria R, Keck PEJ . Antidepressants and suicidal behavior in bipolar disorder. Bipolar Disord 2006; 8: 596–617.
Yerevanian BI, Koek RJ, Mintz J, Akiskal HS . Bipolar pharmacotherapy and suicidal behavior Part 2. The impact of antidepressants. J Affect Disord 2007; 103: 5–11.
Li H, Chen A, Xing G, Wei ML, Rogawski MA . Kainate receptor-mediated heterosynaptic facilitation in the amygdala. Nat Neurosci 2001; 4: 612–620.
Wisden W, Seeburg PH . A complex mosaic of high-affinity kainate receptors in rat brain. J Neurosci 1993; 13: 3582–3598.
Lerma J . Kainate receptor physiology. Curr Opin Pharmacol 2006; 6: 89–97.
Bortolotto ZA, Nistico R, More JC, Jane DE, Collingridge GL . Kainate receptors and mossy fiber LTP. Neurotoxicology 2005; 26: 769–777.
Braga MF, Aroniadou-Anderjaska V, Li H . The physiological role of kainate receptors in the amygdala. Mol Neurobiol 2004; 30: 127–141.
Rodriguez-Moreno A, Herreras O, Lerma J . Kainate receptors presynaptically downregulate GABAergic inhibition in the rat hippocampus. Neuron 1997; 19: 893–901.
Frerking M, Schmitz D, Zhou Q, Johansen J, Nicoll RA . Kainate receptors depress excitatory synaptic transmission at CA3 → CA1 synapses in the hippocampus via a direct presynaptic action. J Neurosci 2001; 21: 2958–2966.
Melyan Z, Lancaster B, Wheal HV . Metabotropic regulation of intrinsic excitability by synaptic activation of kainate receptors. J Neurosci 2004; 24: 4530–4534.
Melyan Z, Wheal HV, Lancaster B . Metabotropic-mediated kainate receptor regulation of IsAHP and excitability in pyramidal cells. Neuron 2002; 34: 107–114.
Einat H . Modeling facets of mania—new directions related to the notion of endophenotypes. J Psychopharmacol 2006; 20: 714–722.
Cryan JF, Holmes A . The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov 2005; 4: 775–790.
Fisahn A, Contractor A, Traub RD, Buhl EH, Heinemann SF, McBain CJ . Distinct roles for the kainate receptor subunits GluR5 and GluR6 in kainate-induced hippocampal gamma oscillations. J Neurosci 2004; 24: 9658–9668.
Crawley JN . What's Wrong with My Mouse: Behavioral Phenotyping of Transgenic and Knockout Mice, 2nd edn. Wiley: New York, 2007.
El-Ghundi M, O'Dowd BF, George SR . Prolonged fear responses in mice lacking dopamine D1 receptor. Brain Res 2001; 892: 86–93.
Engel SR, Creson TK, Hao Y, Shen Y, Maeng S, Nekrasova T et al. The extracellular signal-regulated kinase pathway contributes to the control of behavioral excitement. Mol Psychiatry; advance online publication 29 January 2008; doi: 10.1038/sj.mp.4002135.
Einat H, Yuan P, Manji HK . Increased anxiety-like behaviors and mitochondrial dysfunction in mice with targeted mutation of the Bcl-2 gene: further support for the involvement of mitochondrial function in anxiety disorders. Behav Brain Res 2005; 165: 172–180.
Maeng S, Zarate Jr CA, Du J, Schloesser R, McCammon J, Chen G et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of AMPA receptors. Biol Psychiatry 2007; 63: 349–352.
Gould TD, Chen G, Manji HK . In vivo evidence in the brain for lithium inhibition of glycogen synthase kinase-3. Neuropsychopharmacology 2004; 29: 32–38.
Du J, Gray NA, Falke CA, Chen W, Yuan P, Szabo ST et al. Modulation of synaptic plasticity by antimanic agents: the role of AMPA glutamate receptor subunit 1 synaptic expression. J Neurosci 2004; 24: 6578–6589.
Mulle C, Sailer A, Perez-Otano I, Dickinson-Anson H, Castillo PE, Bureau I et al. Altered synaptic physiology and reduced susceptibility to kainate-induced seizures in GluR6-deficient mice. Nature 1998; 392: 601–605.
Mulle C, Sailer A, Swanson GT, Brana C, O'Gorman S, Bettler B et al. Subunit composition of kainate receptors in hippocampal interneurons. Neuron 2000; 28: 475–484.
Miczek KA, Maxson SC, Fish EW, Faccidomo S . Aggressive behavioral phenotypes in mice. Behav Brain Res 2001; 125: 167–181.
Mi XJ, Chen SW, Wang WJ, Wang R, Zhang YJ, Li WJ et al. Anxiolytic-like effect of paeonol in mice. Pharmacol Biochem Behav 2005; 81: 683–687.
Suaudeau C, Rinaldi D, Lepicard E, Venault P, Crusio WE, Costentin J et al. Divergent levels of anxiety in mice selected for differences in sensitivity to a convulsant agent. Physiol Behav 2000; 71: 517–523.
Cryan JF, Mombereau C . In search of a depressed mouse: utility of models for studying depression-related behavior in genetically modified mice. Mol Psychiatry 2004; 9: 326–357.
Belmaker RH . Bipolar disorder. N Engl J Med 2004; 351: 476–486.
Goodwin FK, Jamison KR . Manic-Depressive Illness. Oxford University Press: New York, 1990.
McKinney R, Bunney WE . Animal models of depression. I. Review of evidence: implications for research. Arch Gen Psychiatry 1969; 21: 240–248.
Willner P . The validity of animal models for depression. Psychopharmacology (Berl) 1984; 83: 1–16.
Jaskolski F, Coussen F, Mulle C . Subcellular localization and trafficking of kainate receptors. Trends Pharmacol Sci 2005; 26: 20–26.
Jaskolski F, Coussen F, Nagarajan N, Normand E, Rosenmund C, Mulle C . Subunit composition and alternative splicing regulate membrane delivery of kainate receptors. J Neurosci 2004; 24: 2506–2515.
Ren Z, Riley NJ, Garcia EP, Sanders JM, Swanson GT, Marshall J . Multiple trafficking signals regulate kainate receptor KA2 subunit surface expression. J Neurosci 2003; 23: 6608–6616.
Ren Z, Riley NJ, Needleman LA, Sanders JM, Swanson GT, Marshall J . Cell surface expression of GluR5 kainate receptors is regulated by an endoplasmic reticulum retention signal. J Biol Chem 2003; 278: 52700–52709.
Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 2000; 47: 351–354.
Zarate Jr CA, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 2006; 63: 856–864.
Kato T, Kubota M, Kasahara T . Animal models of bipolar disorder. Neurosci Biobehav Rev 2007; 31: 832–842.
Prickaerts J, Moechars D, Cryns K, Lenaerts I, van Craenendonck H, Goris I et al. Transgenic mice overexpressing glycogen synthase kinase 3beta: a putative model of hyperactivity and mania. J Neurosci 2006; 26: 9022–9029.
Roybal K, Theobold D, Graham A, DiNieri JA, Russo SJ, Krishnan V et al. Mania-like behavior induced by disruption of CLOCK. Proc Natl Acad Sci USA 2007; 104: 6406–6411.
Kasahara T, Kubota M, Miyauchi T, Noda Y, Mouri A, Nabeshima T et al. Mice with neuron-specific accumulation of mitochondrial DNA mutations show mood disorder-like phenotypes. Mol Psychiatry 2006; 11: 577–593.
Mazzucchelli C, Vantaggiato C, Ciamei A, Fasano S, Pakhotin P, Krezel W et al. Knockout of ERK1 MAP kinase enhances synaptic plasticity in the striatum and facilitates striatal-mediated learning and memory. Neuron 2002; 34: 807–820.
Selcher JC, Nekrasova T, Paylor R, Landreth GE, Sweatt JD . Mice lacking the ERK1 isoform of MAP kinase are unimpaired in emotional learning. Learn Mem 2001; 8: 11–19.
Ferguson SM, Fasano S, Yang P, Brambilla R, Robinson TE . Knockout of ERK1 enhances cocaine-evoked immediate early gene expression and behavioral plasticity. Neuropsychopharmacology 2006; 31: 2660–2668.
McClung CA, Sidiropoulou K, Vitaterna MH, Takahashi JS, White FJ, Cooper DC et al. Regulation of dopaminergic transmission and cocaine reward by the clock gene. Proc Natl Acad Sci USA 2005; 102: 9377–9381.
Fisahn A, Heinemann SF, McBain CJ . The kainate receptor subunit GluR6 mediates metabotropic regulation of the slow and medium AHP currents in mouse hippocampal neurons. J Physiol 2005; 562: 199–203.
Grabauskas G, Lancaster B, O'Connor V, Wheal H . Protein kinase signaling requirements for metabotropic action of kainate receptors in rat CA1 pyramidal neurons. J Physiol 2006; 7: 7.
Acknowledgements
We thank Ioline Henter for providing outstanding editorial assistance. This work was supported by the Intramural Program of the NIMH.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shaltiel, G., Maeng, S., Malkesman, O. et al. Evidence for the involvement of the kainate receptor subunit GluR6 (GRIK2) in mediating behavioral displays related to behavioral symptoms of mania. Mol Psychiatry 13, 858–872 (2008). https://doi.org/10.1038/mp.2008.20
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2008.20
Keywords
This article is cited by
-
Behavioral analysis of kainate receptor KO mice and the role of GluK3 subunit in anxiety
Scientific Reports (2024)
-
Investigation of genetic loci shared between bipolar disorder and risk-taking propensity: potential implications for pharmacological interventions
Neuropsychopharmacology (2021)
-
Enlightened: addressing circadian and seasonal changes in photoperiod in animal models of bipolar disorder
Translational Psychiatry (2021)
-
Dysfunction of ventral tegmental area GABA neurons causes mania-like behavior
Molecular Psychiatry (2021)
-
Therapeutic approaches employing natural compounds and derivatives for treating bipolar disorder: emphasis on experimental models of the manic phase
Metabolic Brain Disease (2021)