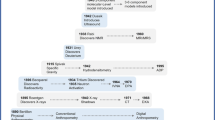
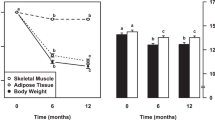
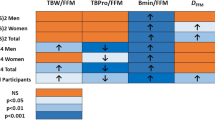
Abstract
The application of advanced methods and techniques and their continuous development enable detailed body composition analyses (BCAs) and modeling of body composition at different levels (e.g., at atomic, molecular, organ-tissue and whole body level). Functional body composition integrates body components into regulatory systems (e.g., on energy balance). Regulation of body weight is closely linked to the mass and function of individual body components. Fat mass is part of the energy intake regulatory feedback system. In addition, fat-free mass (FFM) and fat mass are both determinants of resting energy expenditure (REE). Up to 80% of the variance in energy intake and energy expenditure is explained by body composition. A deviation from normal associations between body components and function suggests a metabolic disequilibrium (e.g., in the REE–FFM relationship or in the plasma leptin–fat mass association) that may occur in response to weight changes and diseases. The concept of functional body composition adds to a more sophisticated view on nutritional status and diseases, as well as to a characterization of biomedical traits that will provide functional evidence relating genetic variants.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Ahima RS (2006). Adipose tissue as an endocrine organ. Obesity 14, 242S–249S.
Assmann G, Schulte H, Seedorf U (2008). Cardiovascular risk assessment in the metabolic syndrome: results from the Prospective Cardiovascular Munster (PROCAM) Study. Int J Obes 32, S11–S16.
Baumgartner RN (2005). Age. In: Heymsfield SB, Lohman TG, Wang Z, Going SB (eds) Human Body Composition, 2nd edn Human Kinetics: Champaign, IL. pp 3–14.
Blaak E, Hul G, Verdich C, Stich V, Martinez JA, Petersen M, et al., The NUTRIGENOB Consortium (2007). Impaired fat-induced thermogenesis in obese subjects: the NUTRIGENOB study. Obesity 15, 653–663.
Bogardus C, Lillioja S, Ravussin E, Abbott W, Zawadzki JK, Young A et al. (1986). Familial dependence of the resting metabolic rate. N Engl J Med 315, 96–100.
Bosy-Westphal A, Eichhorn C, Kutzner D, Illner K, Heller M, Müller MJ (2003). The age-related decline in resting energy expenditure in humans is due to the loss of fat free mass and to alterations in its metabolically active components. J Nutr 133, 2356–2362.
Bosy-Westphal A, Danielzik S, Dörhöfer RP, Later W, Wiese S, Müller MJ (2006). Phase angle from bioelectrical impedance analysis: population reference values by age, sex, and body mass index. JPEN 30, 309–316.
Bosy-Westphal A, Danielzik S, Dörhöfer RP, Piccoli A, Müller MJ (2005). Patterns of bioelectrical impedance vector distribution by body mass index and age: implications for body-composition analysis. Am J Clin Nutr 82, 60–68. Erratum in: Am J Clin Nutr 2005 82, 1358..
Bosy-Westphal A, Müller MJ, Boschmann M, Klaus S, Kreymann G, Lührmann PM et al. (2009). Grade of adiposity affects the impact of fat mass on resting energy expenditure in women. Br J Nutr 101, 474–477.
Bosy-Westphal A, Müller MJ, Göle K, Hitze B, Kossel E, Heller M et al. (2008a). Association between loss in fat mass and adaptive thermogenesis in response to weight loss in obese women. International Symposium on In Vivo Body Composition Studies, 9–12 July, 2008, New York Int J Body Comp Res 6, 75–76.
Bosy-Westphal A, Reinecke U, Schlörke T, Illner K, Kutzner D, Heller M et al. (2004). Effect of organ and tissue masses on resting energy expenditure in underweight, normal weight and obese adults. Int J Obes 28, 72–79.
Bosy-Westphal A, Wolf A, Bührens F, Hitze B, Czech N, Mönig H et al. (2008b). Familial influences and obesity-associated metabolic risk factors contribute to the variation in resting energy expenditure: the Kiel Obesity Prevention Study. Am J Clin Nutr 87, 1695–1701.
Bouchard C, Deriaz O, Perusse L, Tremblay A (1994). Genetics in energy expenditure in humans. In: Bouchard, C (ed) The Genetics of Obesity. CRC Press: Boca Raton. pp 135–146.
Bouchard C, Perusse L (1994). Genetics of obesity: family studies. In: Bouchard, C (ed) The Genetics of Obesity. CRC Press: Boca Raton. pp 79–92.
Chung WK, Leibel R (2008). Considerations regarding the genetics of obesity. Obesity 16 (suppl 3), S33–S39.
Cutler DL, Kaufman S, Freidenberg GE (1991). Insulin-resistant diabetes mellitus and hypermetabolism in mandibuloacral dysplasiea: a newly recognized form of partial lipodystrophy. J Clin Endocrin Metab 73, 1056–1061.
Danforth E, Horton ES, O'Conell M, Sims E, Burger AG, Ingbar SH et al. (1979). Dietary-induced alterations in thyroid hormone metabolism during overfeeding. J Clin Invest 64, 1336–1347.
Deriaz O, Fournier G, Tremblay A, Despres JP, Bouchard C (1992). Lean-body-mass composition and resting energy expenditure before and after long-term overfeeding. Am J Clin Nutr 56, 840–847.
Diaz EO, Prentice AM, Goldberg GR, Murgatroyd PR, Coward WA (1992). Metabolic response to experimental overfeeding in lean and overweight healthy volunteers. Am J Clin Nutr 56, 641–655.
Dulloo AG, Jaquet J (1998). Adaptive reduction in basal metabolic rate in response to food deprivation in humans: a role for feedback signals from fat stores. Am J Clin Nutr 68, 599–606.
Elia M (1992). Organ and tissue contribution to metabolic rate. In: Kinney JM, Tucker HN, (eds) Energy Metabolism: Tissue Determinants and Cellular Corrolaries. Raven Press: New York. pp 61–77.
Farooqi IS, Jebb SA, Langmack G, Lawrence E, Cheetman CH, Prentice AM et al. (1999). Effects of recombinant leptin therapy in a child with congenital leptin deficiency. New Engl J Med 341, 879–884.
Farooqi IS, Matarese G, Lord GM, Keogh JM, Lawrence E, Agwu C et al. (2002). Beneficial effects of leptin on obesity, T cell responsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest 110, 1093–1103.
Gallagher D, Allen A, Wang Z, Heymsfield S, Krasnow N (2000). Smaller organ tissue mass in the elderly fails to explain lower resting metabolic rate. Ann NY Acad Sci 904, 449–455.
Gallagher D, Elia M (2005). Body composition, organ mass, and resting energy expenditure. In: Heymsfield SB, Lohman TG, Wang Z, Going SB (eds) Human Body Composition, 2nd edn Human Kinetics: Champaign, IL. pp 219–240.
Goulding A, Grant AM, Williams SM (2005). Bone and body composition of children and adolescents with repeated forearm fractures. J Bone Miner Res 12, 2090–2096.
Haas V, Onur S, Paul Th, Nutzinger PD, Bosy-Westphal A, Hauer M et al. (2005). Leptin and body weight regulation in patients with anorexia nervosa before and during weight recovery. Am J Clin Nutr 81, 889–896.
Hall KD (2006). Computational model of in vivo human energy metabolism during semistarvation and refeeding. Am J Physiol 291, 23–37.
Heymsfield SB (2002). Heat and life: the ongoing Odyssee. JPEN 26, 319–332.
Heymsfield SB, Greenberg AS, Fujiola K, Dixon RM, Kushner R, Hunt T et al. (1999). Recombinant leptin for weight loss in obese and lean adults. A randomized, controlled, dose-escalation trial. JAMA 282, 1568–1575.
Hwa JJ, Hibaudi L, Compton D, Fawzi AB, Strader CD (1996). Intracerebro-ventricular injection of leptin increases thermogenesis and mobilizes fat metabolism in ob/ob mice. Horm Metab Res 28, 659–663.
Javor ED, Cochran EK, Musso C, Young JR, DePaoli AM, Gorden P (2005). Long-term effect of leptin replacements in patients with generalized lipodystrophy. Diabetes 54, 1994–2002.
Jequier E, Tappy L (1999). Regulation of body weight in humans. Phys Rev 79, 451–480.
Johnstone AM, Murison SD, Duncan JS, Rance KA, Speakman JR (2005). Factors influencing variation in basal metabolic rate include fat-free mass, fat mass, age, and circulating thyroxine but not sex, circulating leptin, or triiodothyronine. Am J Clin Nutr 82, 941–948.
Keys A, Brozek J, Henschel A, Mickelsen O, Taylor HL (1950). Biology of Human Starvation. University of Minnesota Press: Minneapolis.
Keys A, Taylor HL, Grande F (1973). Basal metabolism and age in adult man. Metabolism 22, 579–587.
Klein S, Allison DB, Heymsfield SB, Kelley DE, Leibel RL, Nonas C et al. (2007). Waist circumference and cardiometabolic risk: a consensus statement from Shaping America's Health: Association for Weight Management and Obesity Prevention; NAASO, The Obesity Society; the American Society for Nutrition; and the American Diabetes Association. Am J Clin Nutr 85, 1197–1202.
Klein S, Horowitz JF, Landt M, Goodrick SJ, Mohamed-Ali V, Coppack SW (2000). Leptin production during early starvation in lean and obese women. Am J Physiol 278, E280–E284.
Korth O, Bosy-Westphal A, Zschoche P, Glüer CC, Heller M, Müller MJ (2007). Influence of methods used in body composition analysis on the prediction of resting energy expenditure. Eur J Clin Nutr 61, 582–589.
Kosmiski L, Bessesen DH, Stotz SA, Koeppe JR, Horton TJ (2007). Short-term overfeeding increases resting energy expenditure in patients with HIV-lipodystrophy. Am J Clin Nutr 86, 1009–1015.
Krakoff J, Ma L, Kobes S, Knowler WC, Hanson RL, Bogardus C et al. (2008). Lower metabolic rate in individuals heterozygous for either a frameshift or a functional missense MC4R variant. Diabetes 57, 3267–3272.
Lammert O, Grunner N, Faber P, Schroll-Björnsbo K, Dich J, Larsen LO et al. (2000). Effects of isoenergetic overfeeding of either carbohydrate or fat in young men. Br J Nutr 84, 233–245.
Landsberg L, Young JB (1984). The role of the sympathoadrenal system in modulating energy expenditure. Clin Endocrinol Metab 13, 475–499.
Later W, Bosy-Westphal A, Hitze B, Kossel E, Glüer CC, Heller M et al. (2008). No evidence for mass-dependency of specific metabolic rate in healthy humans. Am J Clin Nutr 88, 1004–1009.
Leibel RL (2002). The role of leptin in the control of body weight. Nutr Rev 60, S15–S19.
Leibel RL, Rosenbaum M, Hirsch J (1995). Changes in energy expenditure resulting from altered body weight. New Engl J Med 332, 621–628.
Müller MJ (2007). Malnutrition and hypermetabolism in patients with liver cirrhosis. Am J Clin Nutr 85, 1167–1168.
Müller MJ, Bosy-Westphal A, Geisler C, Onur S (2005). Physiological vs pathological changes of nutritional status over life time. In: Lochs, H, Thomas, DR (eds) Home Care Enteral Feeding Nestle Nutrition Workshop Series Clinical & Performance Program. Vol. 10 Karger AG: Basel. pp 31–43.
Müller MJ, Bosy-Westphal A, Klaus S, Kreymann G, Lührmann P, Neuhäuser-Berthold M et al. (2004). World Health Organization equations have shortcomings for predicting resting energy expenditure in persons from a modern, affluent population: generation of a new reference standard from a retrospective analysis of a German database of resting energy expenditure. Am J Clin Nutr 80, 1379–1390.
Müller MJ, Bosy-Westphal A, Kutzner D, Heller M (2002). Metabolically active components of fat free mass and resting energy expenditure in humans: recent lessons from imaging technologies. Obes Rev 3, 113–122.
Müller MJ, Böttcher J, Selberg O, Weselmann S, Böker KHW, Schwarze M et al. (1999). Hypermetabolism in clinically stable patients with liver cirrhosis. Am J Clin Nutr 69, 1194–1201.
Nelson KM, Weinsier RL, Long CL, Schutz Y (1992). Prediction of resting energy expenditure from fat free mass and fat mass. Am J Clin Nutr 56, 848–856.
Onur S, Haas V, Bosy-Westphal A, Hauer M, Paul T, Nutzinger D et al. (2005). L-tri-iodothyronine is a major determinant of resting energy expenditure in underweight patients with anorexia nervosa and during weight gain. Eur J Endocrinol 152, 179–184.
Oral EA, Simha V, Ruiz E, Andewelt A, Premkumar A, Snell P et al. (2002). Leptin-replacement therapy for lipodystrophy. New Engl J Med 346, 570–578.
Ortega E, Pannacciulli N, Bogardus C, Krakoff J (2007). Plasma concentrations of free triiodothyronine predict weight change in euthyroid persons. Am J Clin Nutr 85, 440–445.
Ostlund Jr RE, Yang JW, Klein S, Gingerich R (1996). Relation between plasma leptin concentration and body fat, gender, diet, age and metabolic covariates. J Clin Endocrinol Metab 81, 3909–3913.
Peng S, Plank LD, McCall JL, Gillanders LK, McIllroy K, Gane EJ (2007). Body composition, muscle function, and energy expenditure in patients with liver cirrhosis: a comprehensive study. Am J Clin Nutr 85, 1257–1266.
Petersen KF, Befroy D, Dufuour S, Dziura J, Ariyan C, Rothman DL et al. (2003). Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science 300, 1140–1142.
Pichard C, Kyle UG, Bracco D, Slosman DO, Morabia A, Schutz Y (2000). Reference values of fat free and fat masses by bioelectrical impedance analysis in 3393 healthy subjects. Nutrition 16, 245–254.
Porter RK, Andrews JF (1998). Effects of leptin on mitochondrial ‘proton leak’ and uncoupling proteins: implications for mammalian energy metabolism. Proc Nutr Soc 5, 455–460.
Prentice AM, Black AE, Coward WA, Cole TJ (1996). Energy expenditure in overweight and obese adults in affluent societies: an analysis of 319 doubly-labelled water measurements. Eur J Clin Nutr 50, 93–97.
Prentice AM, Moore SE, Collinson AC, O'Conell MA (2002). Leptin and undernutrition. Nutr Rev 60, S56–S67.
Puri V, Czech MP (2008). Lipid droplets: FSP27 knockout enhances their sizzle. J Clin Invest 118, 19–22.
Ravussin E, Bogardus C (1989). Relationship of genetics, age, physical fitness to daily energy expenditure and fuel utilisation. Am J Clin Nutr 49, 968–975.
Ravussin E, Schutz Y, Acheson KJ, Dusmet M, Bourquin L, Jéquier E (1985). Short-term, mixed-diet overfeeding in man: no evidence for ‘luxuskonsumption’. Am J Physiol 249, E470–E477.
Rosenbaum M, Goldsmith R, Bloomfield D, Magnano A, Weimer L, Heymsfield S et al. (2005). Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest 115, 3579–3586.
Rosenbaum M, Hirsch J, Gallagher D, Leibel RL (2008b). Long-term persistence of adaptive thermogenesis in subjects who gave maintained a reduced body weight. Am J Clin Nutr 88, 906–912.
Rosenbaum M, Hirsch J, Murphy E, Leibel RL (2000). Effects of changes in body weight on carbohydrate metabolism, catecholamine excretion, and thyroid hormones. Am J Clin Nutr 71, 1421–1432.
Rosenbaum M, Murphy E, Heymsfield S, Matthews D, Leibel R (2002). Low dose leptin administration reverses effects of sustained weight-reduction on energy expenditure and circulating concentrations of thyroid hormones. J Clin Endocrinol Metab 87, 2391–2394.
Rosenbaum M, Nicholson M, Hirsch J, Murphy E, Chu F, Leibel R (1997). Effect of weight change on plasma leptin concentration and energy expenditure. J Clin Endocrinol Metab 82, 3647–3654.
Rosenbaum M, Sy M, Pavlovich K, Leibel RL, Hirsch J (2008a). Leptin reverses weight-loss-induced changes in regional neural activity responses to visual food stimuli. J Clin Invest 118, 2583–2591.
Schoeller DA (2001). The importance of clinical research: the role of thermogenesis in human obesity. Am J Clin Nutr 73, 511–516.
Schulz LO, Schoeller DA (1994). A compilation o daily energy expenditures and body weights in healthy humans. Am J Clin Nutr 60, 676–681.
Schwarz MW, Woods SC, Seeley RJ, Barsh GS, Baskin D, Leibel RL (2003). Is the energy homeostasis system inherently biased toward weight gain? Diabetes 52, 232–238.
Selberg O, Böttcher J, Pirlich M, Schwarze M, Müller MJ (1999). Clinical significance and correlates of whole body potassium status in patients with liver cirrhosis. Hepatol Res 16, 36–48.
Selberg O, Böttcher J, Tusch G, Pichlmayr R, Henkel E, Müller MJ (1997). Identification of high- and low-risk patients before liver transplantation: a prospective cohort study of nutritional and metabolic parameters in 150 patients. Hepatology 25, 652–657.
Shen W, St-Onge M-P, Wang Z, Heymsfield SB (2005). Study of body composition-an overview. In: Heymsfield SB, Lohman TG, Wang Z, Going SB (eds) Human Body Composition, 2nd edn Human Kinetics: Champaign, IL. pp 3–14.
Solinas G, Summermatter S, Manieri D, Gubler M, Pirola L, Wymann MP et al. (2004). The direct effect of leptin on skeletal muscle thermogenesis is mediated by substrate cycling between de nocvo lipogenesis and lipid oxidation. FEBS Lett 577, 539–544.
Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O et al. (2008). Dynamics of fat cell turnover in humans. Nature 453, 783–787.
Stefan N, Kantartzis K, Machann J, Schick F, Thamer C, Rittig K et al. (2008). Identification and characterization of metabolically benign obesity in humans. Ann Intern Med 168, 1609–1616.
Summermatter S, Manieri D, Russell AP, Seydoux J, Montani JP, Buchala A et al. (2008). Thrifty metabolism that favors fat storage after caloric restriction: a role for skeletal muscle phosphatiylinositol-3-kinase activity and AMP-activated protein kinase. FASEB J 22, 774–778.
Tchoukalova YD, Koutsari C, Karpyak MV, Votruba SB, Wendland E, Jensen MD (2008). Subcutaneous adipocyte size and body fat distribution. Am J Clin Nutr 87, 56–63.
Wang J-L, Chinookoswong N, Yin S, Shi Z-Q (2000a). Calorigenic actions of leptin are additive to, but not dependent on, those of thyroid hormones. Am J Physiol 279, E1278–E1285.
Wang Z, Heshka S, Gallagher D, Boozer CN, Kotler DP, Heymsfield SB (2000b). Resting energy expenditure-fat-free mass relationship: new insight provided by body composition modeling. Am J Physiol 279, E539–E545.
Weinsier R (2001). Etiology of obesity: methodological examination of the set-point theory. JPEN 25, 103–110.
Weinsier R, Schutz Y, Bracco D (1992). Re-examination of the relationship of resting metabolic rate to fat-free mass and to the metabolically active components of fat-free mass in humans. Am J Clin Nutr 55, 790–794.
Weinsier RL, Hunter GR, Desmond RA, Byrne NM, Zuckerman PA, Darnell BE (2002). Free-living activity energy expenditure in women successful and unsuccessful at maintaining a normal body weight. Am J Clin Nutr 75, 499–504.
Weinsier RL, Hunter GR, Zuckerman PA, Redden DT, Darnell BE, Larson DE et al. (2000). Energy expenditure and free-living physical activity in black and white women: comparison before and after weight loss. Am J Clin Nutr 71, 1138–1146.
Westerterp-Platenga MS, Saris WHM, Hukshorn CJ, Campfield LA (2001). Effects of weekly administration of pegylated recombinant human OB protein on appetite profile and energy metabolism in obese men. Am J Clin Nutr 74, 426–434.
Weyer C, Foley JE, Bogardus C, Tataranni PA, Pratley RE (2000). Enlarged subcutaneous abdominal adipocytes size, but not obesity itself, predicts type II diabetes independent of insulin resistance. Diabetologia 43, 1498–1506.
Weyer C, Vozarova B, Ravussin E, Tataranni PA (2001). Changes in energy metabolism in response to 48 h of overfeeding and fasting in Caucasians and Pima Indians. Int J Obes 25, 593–600.
Widman RP, Muntner P, Reynolds K, McGinn A, Rajpathak S, Wylie-Rosett J et al. (2008). The obese without cardio-metabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering. Ann Intern Med 168, 1617–1624.
Wolfe RR (2006). The underappreciated role of muscle in health and disease. Am J Clin Nutr 84, 475–482.
Acknowledgements
This study was funded by grants of the Deutsche Forschungsgemeinschaft (DFG Mü 714/8–3) and the Bundesministerium für Bildung und Forschung (BMBF ‘Krankheitsprävention durch Ernährung: Nahrungsfette und Stoffwechsel, Genvariabilität,-regulation, -funktion und funktionelle Lebensmittelinhaltsstoffe’).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Müller, M., Bosy-Westphal, A., Later, W. et al. Functional body composition: insights into the regulation of energy metabolism and some clinical applications. Eur J Clin Nutr 63, 1045–1056 (2009). https://doi.org/10.1038/ejcn.2009.55
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2009.55
Keywords
This article is cited by
-
Being a scientist
European Journal of Clinical Nutrition (2023)
-
Underweight but not underfat: is fat-free mass a key factor in constitutionally thin women?
European Journal of Clinical Nutrition (2021)
-
Is constitutional thinness really different from anorexia nervosa? A systematic review and meta-analysis
Reviews in Endocrine and Metabolic Disorders (2021)
-
Human body composition: yesterday, today, and tomorrow
European Journal of Clinical Nutrition (2018)
-
Energy expenditure—body size associations: molecular coordination
European Journal of Clinical Nutrition (2018)