Abstract
Monoclonal antibodies (mAb) are emerging as one of the major class of therapeutic agents in the treatment of many human diseases, in particular in cancer and immunological disorders. To date, 28 mAb have been approved by the United States Food and Drug Administration for clinical applications. In addition, several hundreds of mAb are being developed clinically by many biotech and pharmaceutical companies for various disease indications. Many challenges still remain, however, and the full potential of therapeutic antibodies has yet to be realized. With the advancement of antibody engineering technologies and our further understanding of disease biology as well as antibody mechanism of action, many classes of novel antibody formats or antibody derived molecules are emerging as promising new generation therapeutics. These new antibody formats or molecules are carefully designed and engineered to acquire special features, such as improved pharmacokinetics, increased selectivity, and enhanced efficacy. These new agents may have the potential to revolutionize both our thinking and practice in the efforts to research and develop next generation antibody-based therapeutics.
Similar content being viewed by others
Introduction
One of the major milestones in the history of antibody research and development was the invention of hybridoma technology to create monoclonal antibodies (mAb) in 1975 by Georges Kohler and Cesar Milstein1, who were awarded a Nobel Prize in 1984. In 1986, OKT3, the first antibody derived from mouse hybridoma, was approved by the United States Food and Drug Administration (FDA) for use in patients to prevent transplant rejections. The mouse hybridoma derived antibody, however, can be recognized by the human immune system as foreign and induce human anti-mouse antibody (HAMA) response, resulting in short half-life, reduced efficacy, and in some cases increased toxicity in patients. To this end, various antibody discovery and engineering technologies have been developed in order to reduce the immunogenicity of mouse antibody: for example, antibody chimerization2 and humanization3, 4, using recombinant DNA technology were created in 1980s, by replacing portions of murine antibody with the human counterparts. Further, technologies to generate fully human antibodies, such as phage display libraries5 and transgenic mice6, 7, 8, were established in early 1990's. The first chimeric and the first humanized antibodies were approved by the FDA for human use in 1993 and 1997, respectively. Since 2002, seven fully human antibodies generated from phage display and transgenic mice have been approved for therapy applications. These genetically engineered antibodies have proven to be much less immunogenic in patients across various disease indications9. Today, a total of 28 therapeutic antibodies have been approved by the FDA for marketing in the United States (see Table 1). In addition, four other mAb are available for human use in non-US markets. Worldwide sales of therapeutic mAb have risen dramatically in recent years from about $4.0 billion in 2001 to over $30 billion in 2008. The market of therapeutic mAb represents the fastest growing sector in the pharmaceutical industry.
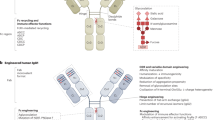
The major focus of antibody engineering technology development in the past three decades has been to reduce immunogenicity of murine antibody and to improve manufacturability. As more therapeutic mAb make their way through research and clinical development, the field has clearly shifted from murine towards humanized and fully human products (see Table 1). Technology advancement in protein production, both in microbial and mammalian cell systems, has enabled us to produce therapeutic antibodies at a level that is more economic than ever. To date, in addition to more than two dozen mAb products either in Phase III clinical trials or awaiting FDA approval, there are several hundred antibodies being tested in early stage clinical trials in a variety of disease indications. Albeit the clinical and commercial success and the current hype among major biotech and pharmaceutical companies continue, many challenges in developing antibody therapeutics remain and the full potential of therapeutic antibodies has yet to be realized. To date, most FDA approved antibodies are full-length unmodified antibodies, which are large proteins with a molecular weight of around 150 kDa. With new genetic engineering technology development and our further understanding of disease biology and mechanisms of action of antibodies, an array of classes of novel antibody formats or antibody derived molecules is emerging as promising new generation therapeutics. These new antibody formats or molecules are carefully designed and engineered to acquire special features, such as improved pharmacokinetics, increased selectivity, and enhanced efficacy.
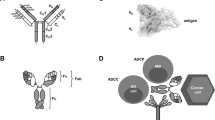
Bispecific antibody (BsAb)
The incurrence of a disease is rarely due to a single point of deregulation, rather most likely multiple mechanisms are developed in the process of disease to reinforce its pathogenesis. Quite often a monotherapy targeting a specific node of biology network in the cell cannot eradicate the disease or prevent the disease from recurrence. An obvious strategy to overcome disease resistance to a single therapy is to simultaneously attack multiple components of the cellular pathways by a combination of drugs that act on different targets and/or mechanisms. This has been greatly validated in cancer treatment as most of the chemotherapy regimens are comprised of a combination of several cytotoxic agents. The combinational strategy has also been shown to be efficacious when combining low molecular weight drugs with therapeutic antibodies, as well as simultaneously administrating two or more therapeutic antibodies. For examples, the combination of cetuximab (Erbitux®, anti-epidermal growth factor receptor, EGFR) and bevacizumab (Avastin®, anti-vascular endothelial growth factor, VEGF) in the treatment of metastatic colorectal cancer10, and rituximab (Rituxan®, anti-CD20) and epratuzumab (anti-CD22) in Non-Hodgkin's lymphoma patients11, have both demonstrated the benefits of antibody combination, when compared to a single antibody therapy, in preliminary clinical studies. However, several critical issues, including high development cost, significant development timelines, and complex regulatory approval path, present high hurdles to developing two individual therapeutic mAb for combination therapy in the future. A promising alternative to antibody combination is to create a bispecific antibody (BsAb), a single antibody molecule capable of strong and specific binding simultaneously with two different target antigens12. Since the conception of the BsAb in late 1970's, early research in the field has been heavily focused on the construction of BsAb for effector cell targeting, ie, a BsAb that can bind to both a tumor associated antigen on cancer cells and an activating molecule such as CD3 on T cells and CD16 on NK cells. In this context, the BsAb can not only cross-link the tumor and the effector cells but also simultaneously activate the effector cells, leading to tumor cell killing/lysis13, 14. Recently, a new concept has emerged, which is quickly gaining significant enthusiasm among major biotech and pharmas, that is to create dual-targeting BsAb that is able to simultaneously bind and also modify two disease-relevant targets12.
Based on the mechanisms of action, dual-targeting BsAb can be constructed to bind to different targets of the signaling pathways within the same diseased cells. Simultaneous modifying (eg, blocking or neutralizing) two disease associated targets should provide the benefits of both enhancing the therapeutic efficacy of, and also blocking the compensatory mechanisms associated with, the individual antibody therapy, thus circumventing resistance to monotherapy15, 16, 17. By simultaneously binding to different cell surface targets, BsAb may result in enhanced binding avidity, leading to preferential (strong) binding to only cells that express both targets but not cells that only express a single target, thus fine-tuning the antibody selectivity18. BsAb can also be designed to bind to different targets expressing on different cell populations within the diseased tissues to achieve synergistic therapeutic effects and/or to enhance specific tissue distribution19. Since each binding arm of a BsAb is functionally active independent of the other, the BsAb should be, in theory, able to exert its biological activity towards diseased cells that express either both the target antigens or just one of the two, thus potentially expanding the therapeutic disease indications of the BsAb16, 17. Further, BsAb can be created from two antibodies that bind to different epitopes on the same target (ie, bi-paratopic binding) to enhance binding avidity20 and to increase antibody load on tumor cells for enhanced effector functions, such as antibody dependent cellular cytotoxicity (ADCC) and/or complement dependent cytotoxicity (CDC)17. It has been demonstrated that, bi-paratopic BsAb, by simultaneously binding to two different epitopes on the same target molecule, could even potentially acquire new functionality that could not be achieved with the parent antibodies when used alone or in combination20.
The development of molecular engineering technologies in the past years has enabled us to design and construct various formats of recombinant BsAb. Early BsAb research and engineering have been concentrated on the creation of bispecific fragments, and significant progress has been made in the past two decades in this field21, 22. BsAb fragments do not require glycosylation, thus they can be produced in high yield in microbia such as bacteria. BsAb fragments are smaller than full length IgGs, so they may have better solid tumor penetration rates. However, their small size and lack of an intact Fc also result in their being cleared rapidly from circulation, leading to a short in vivo half-life. Recently there has been an increased interest on the design and construction of IgG-like BsAb23, 24. These molecules contain an intact Fc, which endows them with the effector functions such as ADCC and CDC, and a half-life of normal IgG, but permute variable domain organizations to endow them with bi-specificity, and in many cases tetravalent binding16, 17, 24. The engineering and application of various BsAb formats have been reviewed extensively21, 22, 23, 24, 25, 26.
A major technological obstacle in the successful development of BsAb has been the difficulty of producing the materials in sufficient quality and quantity for both preclinical and clinical studies. The major challenge in the development of IgG-like BsAb is to construct a recombinant molecule with good pharmaceutical properties comparable to those of the conventional mAb, such as good molecule characteristics (eg, homogeneity, stability, low aggregation propensity, etc), and ease of manufacturing and downstream processing including high level productivity, simple purification process and no special need in formulation. On the biology front, it has long been known that not every single combination of cytotoxics or other disease modifying agents would necessarily lead to additive or synergistic therapeutic effect. Developing highly effective BsAb will require clear elucidation and understanding of the molecular details in the aberrant signaling pathways that lead to various diseases to guide the selection of the target pairs for co-targeting.
Antibody drug conjugates (ADC)
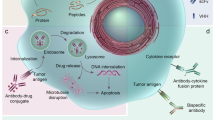
Another new approach in therapeutic antibody development is to use the target specificity of an antibody to deliver therapeutic payloads, typically radioactive isotopes, chemotherapeutic drugs or toxins, to target cells. This is particularly useful for therapeutic antibodies in oncology, the area which sees the most growth of mAb treatments. The non-specific toxic side-effect of chemotherapy on normal tissues is a major issue that severely limits the applications of chemotherapeutic drugs in the clinic. Antibody conjugates can deliver the toxin or chemotherapeutic drugs to specific tumor tissues, thus reducing systemic toxicity and increasing efficacy. Three antibody drug conjugates (ADCs) have been approved by the FDA: gemtuzumab ozogamicin (Mylotarg®, see Table 1, footnote 1, for recent development of the conjugate), a humanized anti-CD33 antibody conjugated to calicheamicin for acute myeloid leukemia27, ibritumomab tiuxetan (Zevalin®), an 90Y-labeled murine anti-CD20 antibody, and tositumomab (Bexxar®), an 131I-labeled murine anti-CD20 antibodies, for non-Hodgkin's lymphoma28. There is clearly a significantly revived interest in the field at this time, as many major biotech and pharmas are intensifying their efforts in promoting ADCs in both discovery research and clinical trials29. For example, Genentech is performing multiple clinical studies with trastuzumab-DM1 (T-DM1), an anti-HER2 mAb-maytansinoid drug conjugate, which had showed greater antitumor activity compared with nonconjugated trastuzumab (Herceptin®, anti-HER2) while maintaining selectivity for HER2-overexpressing tumor cells30. Genentech initiated a Phase III study evaluating T-DM1 for second-line advanced HER2-positive breast cancer in 2009. On July 6, 2010, Genentech submitted a Biologics License Application to the US FDA for T-DM1 in people with advanced HER2-positive breast cancer who have previously received multiple HER2-targeted medicines and chemotherapies. Another ADC in pivotal clinical trial is Brentuximab Vedotin (SGN-35), an anti-CD30 mAb linked to monomethyl auristatin E (MMAE) for Hodgkin lymphoma, from Seattle Genetics. Currently, there are over a dozen of other ADCs, for example, SGN-33 (anti-CD33-MMAE), inotuzumab (anti-CD22)-ozogamicin, IMGN242 [HuC242 (anti-CanAg)-DM4], CDX-011 (anti-GPNMB-MMAE), MDX1203 (anti-CD70-duocarmycin) and MEDI-547 (anti-EphA2-MMAE), AVE9633 (anti-CD33-DM4), in various stages of clinical trials31, 32.
ADC has three components, the mAb, the cytotoxic payload, and the linker connecting the payload to the antibody. The antibody carrier should be highly specific and efficiently internalized once binding to its target antigen on the cell surface. The desired properties for a toxic payload include high potency towards tumor cells, a suitable functional group for linkage to an antibody, reasonable solubility in aqueous solutions to enable the reaction with antibodies, and prolonged stability in aqueous formulations commonly used for antibodies. The linker between the antibody and the payload should be designed in a manner that ensures ADC stability during storage and in circulation in vivo but allows for a rapid release of the cytotoxic payload in its fully active form once inside the target cells. In the past several years, significant progress has been made in optimizing each of the three components of an ADC. Less immunogenic and more selective high affinity antibody carriers have been designed and selected. Toxic payloads have evolved from radio isotope and conventional chemotherapeutics to more potent cytotoxic agents, such as calicheamicin, maytansinoids and auristatins. Several types of cleavable (labile) or non-cleavable (stable) linkers, for example, disulfide linkers and acid- and peptidase-labile linkers, have been developed31. The conjugation technologies have advanced to a point where both the site and stoichiometry of drug attachment to the carrier antibody can be controlled. In the near future, the research focus of the area will be to identify even more potent payloads and to develop better conjugation strategies including further improvement in linker design and conjugation chemistry and efficiency. Other areas that critically need to be addressed include establishment of analytic platforms for manufacturing and process development (chemistry, manufacturing and control, CMC) and clinical pharmacokinetic/pharmacodynamic assays, and development of preclinical toxicology and pharmacology assessment protocols to satisfy the regulatory and safety requirement.
Antibody with modified Fc functions (Fc engineering)
In addition to the direct effect of binding to an antigen, antibodies can mediate a variety of “effector” functions such as ADCC and CDC, via their Fc regions. By fixing complement or interacting with the Fc receptors (FcRs) thus activating immune cells such as NK cells, macrophages, and T cells, the antibody can mediate additional cell killing against target cells. These effector mechanisms are particularly relevant when the antibodies are used to treat cancer and certain inflammatory diseases. ADCC as part of the mechanisms of action for therapeutic antibodies has been strongly implicated in several clinical trials. For example, a better clinical response to rituximab is observed in non-Hodgkin's lymphoma patients carrying an IgG FcγRIIIa of V158 allotype, an allotype with higher affinity binding to the Fc region of an IgG, compared to that in patients who carry the F158 allotype33. Similarly, patients carrying the 158 VV genotype of FcγRIIIa were also associated with a better clinical response to trastuzumab34 and cetuximab35. Based on these clinical observations, it is plausible to further enhance the therapeutic efficacy of a mAb by optimizing (increasing) its Fc interaction with the FcRs on effector cells, via molecular engineering and/or manufacturing process modification36, 37, 38.
By combining various molecular engineering methods, including alanine scanning, site-directed mutagenesis, computational structure-based design/algorithm and experimental selections36, 37, 39, 40, a large set of Fc variants has been generated that provides a spectrum of FcγR binding profiles. Several variants have been identified that provide up to 100-fold greater affinity for FcγRIIIa, resulting in an enhanced ADCC, for up to two to three logs higher, than what can be achieved by the wild-type antibody38, 41. For example, a Fc-engineered anti-CD19 antibody, with 100–1000 fold increased ADCC, has shown more potent antitumor activity than its IgG1 analogue in prophylactic and established mouse xenograft models42. In addition to ADCC, an IgG1/IgG3 chimeric Fc, constructed via alternative domain shuffling, has demonstrated markedly increased CDC activity both in vitro and in vivo in a cynomolgus monkey model43. Albeit of all the encouraging observations, specific design and selection of Fc variants with preferentially enhanced binding to individual FcγR, either the activating FcγRIIIa, FcγRIIa, and FcγRIa, or the inhibitory FcγRIIb, still remain a challenge. It also remains to be seen whether it is possible to fine-tune the Fc domain of a defined mAb agent to selectively activate (or inhibit) a sub-population of immune cells and/or to optimize the CDC activity for intended therapeutic application.
The glycosylation of the antibody Fc domain also has a major influence on its binding affinity for FcγRs. It is well known that the carbohydrates at the position Asn297 is critical for FcγR binding. The presence of fucose and its content at this position can negatively influence ADCC activity of a mAb. To this end, new cell lines capable of producing defucosylated mAb have been established, such as CHO cell lines that are genetically engineered (knock-out) to delete the FUT8 gene coding for the enzyme α-1,6-fucosyltransferase44. Alternatively, CHO cell lines that over-express a recombinant β-1,4-N-acetylglucosaminyltransferase III (GnTIII) have also been established. Production in these cell lines resulted in antibodies enriched in bisected and non-fucosylated oligosaccharides45. Antibodies produced in these modified cell lines have been shown to be more potent than their counterparts produced in the wild-type CHO cell lines in mediating ADCC towards target cells44, 45. Similar approaches have also been attempted in non-mammalian expression systems, including yeast, plants, and moss, in parallel to engineering away non-human (thus potentially immunogenic) glycoforms46, 47, 48. Other glycoform modifications, such as sialylation of the Fc carbohydrate, has also been suggested as a biological mechanism for regulation of FcγR affinity and cytotoxicity49.
In addition to its effector activities, another major function of the antibody Fc domain is to bind to the neonatal Fc receptor (FcRn), the major mechanism responsible for the long circulation half-life of an antibody, compared to other proteins of similar size. A long serum half-life is generally desirable as it would reduce the frequency of dosing, thus potentially reducing both the inconvenience and the total cost of the treatments. It has been demonstrated in several reports that various mutations within the Fc domain of an IgG1 led to significantly improved binding to FcRn at pH 6.0 (but no changes in binding at pH 7.4). The enhanced FcRn binding resulted in increased antibody circulation half-life in cynomolgus monkeys and improved in vivo dosing regimen50, 51, 52, 53, 54, 55. It has been suggested that the kinetics of Fc/FcRn interaction may also have an effect on the extent to which improved binding translates into extended serum half-life56. Besides IgG, a half-life enhanced Fc fragment can also be used as the fusion partner to extend the half-life of antibody fragments, alternative scaffolds, and other protein therapeutics.
Antibody fragments and single domain antibodies
Full length IgG has good in vivo half-life and effector functions, but its large size limits antibody tissue penetration, especially in solid tumor, and complicates manufacturing process. Antibody genetic engineering has enabled us to produce various antibody fragments, with defined size, valency, and desired pharmacokinetics profiles, that retain the binding activity of the full-length molecule, to suit various in vivo applications57. For example, it is relatively straight-forward to create and produce antibody fragments with different size and valence, such as Fab, single chain Fv (scFv)58, diabody59, and minibody60 etc. It has been demonstrated that, in some species, even a single variable antibody domain could bind to antigen with high affinities. Camelids and sharks are two species that naturally express heavy chain only antibodies, called heavy chain antibodies (HcAbs)61 and new antigen receptor antibodies (IgNAR)62, respectively. The single variable domains of their antibodies, called VHH in camelids and V-NAR in sharks, have been engineered to retain high affinities towards a large spectrum of antigens. Recently, single domain antibody (dAb) based on a single human antibody variable domain, VH or VL, has also been generated with high affinity and specificity63. To date, three antibody Fab fragments have been approved for clinical applications, including abciximab (Reopro®, anti-GPIIb/IIIa receptor), ranibizumab (Lucentis®, anti-VEGF) and certolizumab pegol (Cimzia®, anti-tumor necrosis factor α, TNFα). Several single domain antibodies are currently in various stages of clinical development, including two dAbs (anti-TNFα and anti-IL-1R) and three nanobodies (anti-vWF, anti-RANKL, and anti-TNFα), the humanized camelid (llama)-derived VHH domains.
Because of their reduced size, antibody fragments usually penetrate solid tissue much more rapidly and efficiently than the full length IgG, but this advantage is countered by a very short serum half-life that eventually could decrease the overall tissue uptake of these fragments. Several approaches are being explored to increase the serum half-life of antibody fragments. One approach is PEGylation [chemical addition of polyethylene glycol (PEG) to increase the size of the fragments], exemplified by certolizumab pegol, a recently approved anti-TNFα PEGylated Fab fragment64. Other alternative approaches include HSA fusion (fusion of recombinant antibody fragments to human serum albumin)65 and anti-HSA fusion (fusion of antibody fragments to HSA-binding peptides or proteins)66, 67, 68. Multimerization of antibody fragments has also been explored. For example, diabodies, triabodies and tetrabodies have been produced by multimerization of scFvs harboring a short or no linker between the VL and the VH, leading to high molecular weight and multivalent fragments with increased serum half-lives57.
Alternative protein scaffold
Antibodies gain their universal antigen recognition function by the combination of a structurally conserved framework with a spatially defined combining site composed of peptide segments that are hypervariable both in sequence and conformation69, referred to as the complementarity determining regions, or CDRs. Alternative protein scaffolds provide a potential avenue for developing a new class of biotherapeutics. Dozens of small independently folding proteins and domains have been evaluated as antibody mimics in attempts to obtain high affinity binders with therapeutic potential.
Fibronectin is perhaps the most advanced scaffold in development. Adnectin is one of the examples. It is based on the 10th fibronectin type III domain, a highly stable (Tm=90 °C) 94-residue member of the Ig superfamily which does not contain any cysteines. Three loops of the protein can be randomized in similar fashion to the CDRs of a single domain antibody, and the resulting library has been used to select therapeutically useful binders to various targets70. CT-322, a pegylated anti-VEGFR2 adnectin, is currently in Phase II clinical development as an angiogenesis inhibitor in the United States in recurrent glioblastoma multiforme (rGBM) and in a Phase Ib/II study for first line GBM.
Avimers is another advanced case in this category. They are based on 40-amino-acid-long human A-domains, which occur as strings of multiple domains in several cell-surface receptors. Although each of the domains separately has low target-binding activity, in tandem arrays very high avidities can be achieved. C326, a subnanomolar IL-6-blocking avimer has been successfully generated. It showed 0.8 pmol/L IC50 in cell-based assays and was biologically active in two animal models71. C326 has been in phase I clinical trial in adults with Crohn's disease since 2006.
Another alternative scaffold being developed is ankyrin repeat proteins (DARPins), which are designed based on a combination of sequence and structure consensus analyses of one of the largest highly conserved protein families in nature72. A 33 amino acid residue ankyrin repeat (AR) module with seven randomized positions can provide a theoretical diversity of 7.2×107. Different numbers of this module, eg four to six repeats, can be assembled together between the N and C-terminal capping repeats to further increase the theoretical diversity. DARPins with picomolar affinities have been isolated and affinity matured against human EGFR73, as well as eukaryotic kinases JNK2 and p3874, underlining their therapeutic potential as intracellular inhibitors. The lead product MP0112, a DARPin which inhibits all relevant forms of VEGF with high potency, is currently in Phase I/IIa testing in wet age-related macular degeneration (wet AMD) and diabetic macular edema (DME).
In addition, alternative scaffolds based on staphylococcal protein A75, lipocalins76, thioredoxin77 and many others78 have also been explored. Compared to the conventional IgG antibodies, small protein scaffolds may provide several advantages that render them good candidates for the development of therapeutic agents. For example, due to their small size and generally high stability, the scaffold proteins may be more amendable for alternative delivery routes, eg, by inhalation or subcutaneous injection. Further, owing to their single domain structure, the scaffold proteins may represent excellent building blocks for the construction of bi- and multi-specific/valent therapeutics. Taken together, the alternative protein scaffolds have generated high interests and expectations in recent years, and many of the promising scaffolds are under serious research and development. There are several major issues remaining to be addressed, in particular, the potential immunogenicity, the lack of effector functions, and the short circulation half-life in the context of therapeutic use of the scaffold proteins. These issues will no doubt be the focus of the field in the near future.
Intrabody
Antibody is naturally secreted into extracellular space and binds antigen extracellularly, so it is natural that all the targets for current therapeutic antibodies and antibody derivatives are extracellular proteins. Recent advances in antibody engineering have allowed the specific, high affinity interaction of antibodies with antigens to be directed intracellularly through the creation of intracellular antibodies, or so-called “intrabodies”. Intrabody expands the pool of targets for therapeutic antibody beyond the traditional extracellular proteins. The most commonly used intrabodies are in the form of scFv as a single polypeptide. Other antibody formats have also been used, including Fab fragments79, single domain antibodies80, conventional IgG antibodies, and multivalent bispecific fragments (for example, “intradiabody” 81). Intrabodies, through the use of N- or C-terminal tags that encode natural intracellular trafficking signals, can direct the antibody-antigen interaction to a specific cellular compartment82. As a result, intrabodies can exert their biological activities via interfering with the intracellular transport or misdirecting the localization of proteins, as demonstrated in the cases of VEGFR2 intrabody83 and EGFR intrabody84. Intrabodies can also achieve their therapeutic effect by directly interfering with enzyme function intracellularly, as shown in the cases of intrabodies against Akt85 and epithelial tyrosine kinase (Etk)86. Further, intrabodies may function by blocking protein-DNA interactions in the nucleus. A nuclear-targeted intrabody has been developed that binds to cyclin-E, interferes with its function, and inhibits the growth of a breast cancer cell line87.
Intrabodies have been isolated to a diverse array of targets that are involved in the pathogenesis of various human diseases and have demonstrated efficacies in a variety of in vitro and in vivo studies. These include intrabodies that inhibit cancer growth88, HIV infection79, 89, 90, HPV infection91, protein misfolding in neurologic disorders including Huntington's disease, Parkinson's disease, Alzheimer's disease and Prion disease92, and knockout of MHC class I expression in an attempt to prevent graft rejection93. The ER-targeted anti-erbB-2 intrabody has been in phase I clinical trial for cancer treatment94. Most approaches using intrabodies to treat a specific disease are in essence a gene therapy approach, involving the introduction of genes into target cells to correct the aberrant molecular processes responsible for the diseased state. Compared to other gene therapy approaches, such as antisense oligonucleotides (AnO)95 and RNA interference methods (RNAi)96, the intrabody approach may offer several advantages, such as higher specificity, longer half-life, and an ability to target proteins with particular post-translational modifications.
There are two major technical issues associated with the development of intrabodie as therapeutics. One involves the efficiency and specificity of delivery of the intrabody or the genetic material encoding the intrabody to disease sites and the expression of intrabodies inside the target cells at a stable and high enough level needed to obtain a therapeutic effect. Several groups have successfully used adenovirus to deliver intrabodies against cancer-related targets in animal models81, 94. However, the risk of potential genetic modification97, immnogenicity and selectivity of delivery have been the concern for viral-based DNA delivery, though several other approaches are being explored98, 99. Another system for intrabody delivery has been developed that involves transporting the intrabody (at the protein level) across the plasma membrane of a cell using membrane translocating sequence (MTS) or protein transduction domains (PTD)85, 100, 101. However, it still remains to be determined if such protein translocation approaches can obtain a high enough level, activity, and half-life of an intrabody in a target cell to achieve a therapeutic effect, especially in vivo. Another issue associated with the clinical development of intrabodies is the correct folding or stability of intrabodies and their tendency to aggregate when expressed in the reducing environment of the subcellular compartments. Many groups are actively addressing this issue, with most of the studies focusing on isolating functional intrabodies from large libraries using selection conditions mimicking the intracellular environment, creating stable antibody frameworks for intrabody construction, and exploring alternative intrabody formats, eg using Intracellular Antibody Capture (IAC) system102, direct phage to intrabody screening (DPIS) system103, modified yeast two-hybrid system104, or using scFv, single domain antibody80, 86. The lessons learned from all of these studies and progress on other forms of gene therapy should facilitate the development of intrabodies as therapeutics.
Perspectives
Compared to low molecular weight chemical drugs, therapeutic antibodies have high target specificity, lower systemic toxicity, longer half-life, and potentially higher barrier for generic (or biosimilar) competition. The future growth of mAb based therapeutics is still strong. Among all biologics that are being studied in clinical trials, 85% are mAb or antibody based molecules. Antibodies and antibody-based therapeutics consist of more than one third of all new agents currently under both preclinical and clinical development at biotech/pharma companies around the world. Consensus sales forecasts predicts that, by 2014, six out of the world's top 10 best-selling drugs will be therapeutic antibodies or antibody fusion protein, and the total sales of mAb based therapeutics will approach $58 billion dollars105.
There are many challenges remaining in the continuous successful development of mAb therapeutics. Despite the completion of the human genome project more than ten years ago, new therapeutic target discovery/identification and validation, remain elusive and challenging tasks. Competition on a limited number of validated targets is fierce. For example, for the top 8 mostly pursued therapeutic targets, 32 biotech/pharma companies had active mAb development programs in 2009. Novel antibody formats engineered for enhanced therapeutic efficacy, such as BsAb and ADC, may open new target space for the development of mAb therapeutics. To this end, certain targets previously deemed not viable for therapeutic development, for example, targets overexpressed on diseased tissue but not associated with known major pathophysiologic functions for intervention (eg, CD33), may prove to be good candidates for ADC development. Further, there are individual targets (pathways) that may not provide sufficient therapeutic benefit when being modified alone, but if combined with other targets (pathways), could produce additive and/or synergistic activity. These targets thus represent excellent candidates for the development of dual-targeting BsAb therapeutics. Besides the targets, development of new technology platforms in antibody discovery/engineering and production to increase the efficacy of therapeutic mAb, and at the same time, to reduce the cost of the therapy, represent the other critical components in winning the competition. We expect to witness more novel mAb and mAb-like molecules with carefully engineered features, as those discussed in this article, to enter clinical development in the near future. Many issues remain to be carefully researched and addressed, for example, manufacturing and downstream process development (purification and formulation), analytical and quality control, preclinical toxicology and pharmacology studies, regulatory and clinical development pathways, etc, before we see these new antibody formats become bona fide mainstream therapeutics.
References
Kohler G, Milstein C . Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975; 256: 495–7.
Morrison SL, Johnson MJ, Herzenberg LA, Oi VT . Chimeric human antibody molecules: mouse antigen-binding domains with human constant region domains. Proc Natl Acad Sci USA 1984; 81: 6851–5.
Jones PT, Dear PH, Foote J, Neuberger MS, Winter G . Replacing the complementarity-determining regions in a human antibody with those from a mouse. Nature 1986; 321: 522–5.
Queen C, Schneider WP, Selick HE, Payne PW, Landolfi NF, Duncan JF, et al. A humanized antibody that binds to the interleukin 2 receptor. Proc Natl Acad Sci USA 1989; 86: 10029–33.
Clackson T, Hoogenboom HR, Griffiths AD, Winter G . Making antibody fragments using phage display libraries. Nature 1991; 352: 624–8.
Taylor LD, Carmack CE, Schramm SR, Mashayekh R, Higgins KM, Kuo CC, et al. A transgenic mouse that expresses a diversity of human sequence heavy and light chain immunoglobulins. Nucleic Acids Res 1992; 20: 6287–95.
Lonberg N, Taylor LD, Harding FA, Trounstine M, Higgins KM, Schramm SR, et al. Antigen-specific human antibodies from mice comprising four distinct genetic modifications. Nature 1994; 368: 856–9.
Green LL, Hardy MC, Maynard-Currie CE, Tsuda H, Louie DM, Mendez MJ, et al. Antigen-specific human monoclonal antibodies from mice engineered with human Ig heavy and light chain YACs. Nat Genet 1994; 7: 13–21.
Hwang WYK . Foote J . Immunogenicity of engineered antibodies. Methods 2005; 36: 3–10.
Lee JJ, Chu E . An update on treatment advances for the first-line therapy of metastatic colorectal cancer. Cancer J 2007; 13: 276–81.
Leonard JP, Schuster SJ, Emmanouilides C, Couture F, Teoh N, Wegener WA, et al. Durable complete responses from therapy with combined epratuzumab and rituximab: final results from an international multicenter, phase 2 study in recurrent, indolent, non-Hodgkin lymphoma. Cancer 2008; 113: 2714–23.
Marvin JS, Zhu Z . Bispecific antibodies for dual-modality cancer therapy: Killing two signaling cascades with one stone. Curr Opin Drug Discov Devel 2006; 9: 184–93.
Garrido MA, Valdayo MJ, Winkler DF, Titus JA, Hecht TT, Perez P, et al. Targeting human T-lymphocytes with bispecific antibodies to react against human ovarian carcinoma cells growing in nu/nu mice. Cancer Res 1990; 50: 4227–32.
Baeuerle PA, Reinhardt C . Bispecific T-cell engaging antibodies for cancer therapy. Cancer Res 2009; 69: 4941–4.
Lu D, Jimenez X, Zhang H, Wu Y, Bohlen P, Witte L, et al. Complete inhibition of vascular endothelial growth factor (VEGF) activities with a bifunctional diabody directed against both VEGF kinase receptors, fms-like tyrosine kinase receptor and kinase insert domain-containing receptor. Cancer Res 2001; 61: 7002–8.
Lu D, Zhang H, Ludwig D, Persaud A, Jimenez X, Burtrum D, et al. Simultaneous blockade of both the epidermal growth factor receptor and the insulin-like growth factor receptor signaling pathways in cancer cells with a fully human recombinant bispecific antibody. J Biol Chem 2004; 279: 2856–65.
Lu D, Zhang H, Koo H, Tonra J, Balderes P, Prewett M, et al. A fully human recombinant igg-like bispecific antibody to both the epidermal growth factor receptor and the insulin-like growth factor receptor for enhanced antitumor activity. J Biol Chem 2005; 280: 19665–72.
Robinson MK, Hodge KM, Horak E, Sundberg AL, Russeva M, Shaller CC, et al. Targeting ErbB2 and ErbB3 with a bispecific single-chain Fv enhances targeting selectivity and induces a therapeutic effect in vitro. Br J Cancer 2008; 99: 1415–25.
Shen J, Vil MD, Prewett M, Damoci C, Zhang H, Li H, et al. Development of a fully human anti-PDGFRbeta antibody that suppresses growth of human tumor xenografts and enhances antitumor activity of an anti-VEGFR2 antibody. Neoplasia 2009; 11: 594–604.
Lu D, Kotanides H, Jimenez X, Zhou Q, Persaud K, Bohlen P, et al. Acquired antagonistic activity of a bispecific diabody directed against two different epitopes on vascular endothelial growth factor receptor 2. J Immunol Methods 1999; 230: 159–71.
Todorovska A, Roovers RC, Dolezal O, Kortt AA, Hoogenboom HR, Hudson PJ . Design and application of diabodies, triabodies and tetrabodies for cancer targeting. J Immunol Methods 2001; 248: 47–66.
Carter P, Ridgway J, Zhu Z . Toward the production of bispecific antibody fragments for clinical applications. J Hematother 1995; 4: 463–70.
Carter P . Bispecific human IgG by design. J Immunol Methods 2001; 248: 7–15.
Marvin JS, Zhu Z . Recombinant approaches to IgG-like bispecific antibodies. Acta Pharmacol Sin 2005; 26: 649–58.
Kipriyanov SM, Le GF . Recent advances in the generation of bispecific antibodies for tumor immunotherapy. Curr Opin Drug Discov Devel 2004; 7: 233–42.
Alt M, Muller R, Kontermann RE . Novel tetravalent and bispecific IgG-like antibody molecules combining single-chain diabodies with the immunoglobulin gamma1 Fc or CH3 region. FEBS Lett 1999; 454: 90–4.
Stasi R, Evangelista ML, Buccisano F, Venditti A, Amadori S . Gemtuzumab ozogamicin in the treatment of acute myeloid leukemia. Cancer Treat Rev 2008; 34: 49–60.
Silverman DH, Delpassand ES, Torabi F, Goy A, McLaughlin P, Murray JL . Radiolabeled antibody therapy in non-Hodgkin's lymphoma: radiation protection, isotope comparisons and quality of life issues. Cancer Treat Rev 2004; 30: 165–72.
Carter PJ . Potent antibody therapeutics by design. Nat Rev Immunol 2006; 6: 343–57.
Lewis Phillips GD, Li G, Dugger DL, Crocker LM, Parsons KL, Mai E, et al. Targeting HER2-positive breast cancer with trastuzumab-dm1, an antibody-cytotoxic drug conjugate. Cancer Res 2008; 68: 9280–90.
Carter PJ, Senter PD . Antibody-drug conjugates for cancer therapy. Cancer J 2008; 14: 154–69.
Teicher BA . Antibody-drug conjugate targets. Curr Cancer Drug Targets 2009; 9: 982–1004.
Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood 2002; 99: 754–8.
Musolino A, Naldi N, Bortesi B, Pezzuolo D, Capelletti M, Missale G, et al. Immunoglobulin G fragment c receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with her-2/neu-positive metastatic breast cancer. J Clin Oncol 2008; 26: 1789–96.
Lejeune J, Thibault G, Ternant D, Cartron G, Watier H, Ohresser M . Evidence for linkage disequilibrium between fc{gamma}RIIIa-v158f and fc{gamma}riia-h131r polymorphisms in white patients, and for an fc{gamma}riiia-restricted influence on the response to therapeutic antibodies. J Clin Oncol 2008; 26: 5489–91.
Shields RL, Namenuk AK, Hong K, Meng YG, Rae J, Briggs J, et al. High resolution mapping of the binding site on human IgG1 for Fc gamma RI, Fc gamma RII, Fc gamma RIII, and FcRn and design of IgG1 variants with improved binding to the Fc gamma R. J Biol Chem 2001; 276: 6591–604.
Lazar GA, Dang W, Karki S, Vafa O, Peng JS, Hyun L, et al. Engineered antibody Fc variants with enhanced effector function. Proc Natl Acad Sci USA 2006; 103: 4005–10.
Desjarlais JR, Lazar GA, Zhukovsky EA, Chu SY . Optimizing engagement of the immune system by anti-tumor antibodies: an engineer's perspective. Drug Discov Today 2007; 12: 898–910.
Allan B . Variant Fc regions. ed. Applied Molecular Evolution. PCT/US2006/025276. 2010. Ref Type: Patent.
Hansen G, et al. Altered antibody Fc regions and uses thereof. ed. Diversa/Medarex. PCT/US2006/011048. 2010. Ref Type: Patent.
Siberil S, Dutertre CA, Boix C, Bonnin E, Ménez R, Stura E, et al. Molecular aspects of human FcgammaR interactions with IgG: functional and therapeutic consequences. Immunol Lett 2006; 106: 111–8.
Horton HM, Bernett MJ, Pong E, Peipp M, Karki S, Chu SY, et al. Potent in vitro and in vivo activity of an fc-engineered anti-cd19 monoclonal antibody against lymphoma and leukemia. Cancer Res 2008; 68: 8049–57.
Natsume A, In M, Takamura H, Nakagawa T, Shimizu Y, Kitajima K, et al. Engineered antibodies of IgG1/IgG3 mixed isotype with enhanced cytotoxic activities. Cancer Res 2008; 68: 3863–72.
Yamane-Ohnuki N, Kinoshita S, Inoue-Urakubo M, Kusunoki M, Iida S, Nakano R, et al. Establishment of FUT8 knockout Chinese hamster ovary cells: an ideal host cell line for producing completely defucosylated antibodies with enhanced antibody-dependent cellular cytotoxicity. Biotechnol Bioeng 2004; 87: 614–22.
Umana P, Jean-Mairet J, Moudry R, Amstutz H, Bailey JE . Engineered glycoforms of an antineuroblastoma IgG1 with optimized antibody-dependent cellular cytotoxic activity. Nat Biotech 1999; 17: 176–80.
Cox KM, Sterling JD, Regan JT, Gasdaska JR, Frantz KK, Peele CG, et al. Glycan optimization of a human monoclonal antibody in the aquatic plant Lemna minor. Nat Biotechnol 2006; 24: 1591–7.
Li H, Sethuraman N, Stadheim TA, Zha D, Prinz B, Ballew N, et al. Optimization of humanized IgGs in glycoengineered Pichia pastoris. Nat Biotechnol 2006; 24: 210–5.
Nechansky A, Schuster M, Jost W, Siegl P, Wiederkum S, Gorr G, et al. Compensation of endogenous IgG mediated inhibition of antibody-dependent cellular cytotoxicity by glyco-engineering of therapeutic antibodies. Mol Immunol 2007; 44: 1815–7.
Kaneko Y, Nimmerjahn F, Ravetch JV . Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science 2006; 313: 670–3.
Dall'Acqua WF, Kiener PA, Wu H . Properties of human IgG1s engineered for enhanced binding to the neonatal Fc receptor (FcRn). J Biol Chem 2006; 281: 23514–24.
Oganesyan V, Damschroder MM, Woods RM, Cook KE, Wu H, Dall'acqua WF . Structural characterization of a human Fc fragment engineered for extended serum half-life. Mol Immunol 2009; 46: 1750–5.
Yeung YA, Wu X, Reyes AE 2nd, Vernes JM, Lien S, Lowe J, et al. A therapeutic anti-VEGF antibody with increased potency independent of pharmacokinetic half-life. Cancer Res 2010; 70: 3269–77.
Zalevsky J, Chamberlain AK, Horton HM, Karki S, Leung IW, Sproule TJ, et al. Enhanced antibody half-life improves in vivo activity. Nat Biotechnol 2010; 28: 157–9.
Hinton PR, Xiong JM, Johlfs MG, Tang MT, Keller S, Tsurushita N, et al. An engineered human IgG1 antibody with longer serum half-life. J Immunol 2006; 176: 346–56.
Hinton PR, Johlfs MG, Xiong JM, Hanestad K, Ong KC, Bullock C, et al. Engineered human IgG antibodies with longer serum half-lives in primates. J Biol Chem 2004; 279: 6213–6.
Datta-Mannan A, Witcher DR, Tang Y, Watkins J, Wroblewski VJ . Monoclonal antibody clearance. J Biol Chem 2007; 282: 1709–17.
Holliger P, Hudson PJ . Engineered antibody fragments and the rise of single domains. Nat Biotechnol 2005; 23: 1126–36.
Bird RE, Hardman KD, Jacobson JW, Johnson S, Kaufman BM, Lee SM, et al. Single-chain antigen-binding proteins. Science 1988; 242: 423–6.
Holliger P, Prospero T, Winter G . "Diabodies": small bivalent and bispecific antibody fragments. Proc Natl Acad Sci USA 1993; 90: 6444–8.
Quiocho FA . Protein engineering. Making of the minibody. Nature 1993; 362: 293–4.
Hamers-Casterman C, Atarhouch T, Muyldermans S, Robinson G, Hamers C, Songa EB, et al. Naturally occurring antibodies devoid of light chains. Nature 1993; 363: 446–8.
Dooley H, Flajnik MF, Porter AJ . Selection and characterization of naturally occurring single-domain (IgNAR) antibody fragments from immunized sharks by phage display. Mol Immunol 2003; 40: 25–33.
Holt LJ, Herring C, Jespers LS, Woolven BP, Tomlinson IM . Domain antibodies: proteins for therapy. Trends Biotechnol 2003; 21: 484–90.
Blick SK, Curran MP . Certolizumab Pegol: In Crohn's Disease. BioDrugs 2007; 21: 195–201.
Muller D, Karle A, Meissburger B, Höfig I, Stork R, Kontermann RE, et al. Improved pharmacokinetics of recombinant bispecific antibody molecules by fusion to human serum albumin. J Biol Chem 2007; 282: 12650–60.
Nguyen A, Reyes AE 2nd, Zhang M, McDonald P, Wong WL, Damico LA, et al. The pharmacokinetics of an albumin-binding Fab (AB.Fab) can be modulated as a function of affinity for albumin. Protein Eng Des Sel 2006; 19: 291–7.
Holt LJ, Basran A, Jones K, Chorlton J, Jespers LS, Brewis ND, et al. Anti-serum albumin domain antibodies for extending the half-lives of short lived drugs. Protein Eng Des Sel 2008; 21: 283–8.
Rothbauer U, Zolghadr K, Tillib S, Nowak D, Schermelleh L, Gahl A, et al. Targeting and tracing antigens in live cells with fluorescent nanobodies. Nat Methods 2006; 3: 887–9.
Skerra A . Imitating the humoral immune response. Curr Opin Chem Biol 2003; 7: 683–93.
Getmanova EV, Chen Y, Bloom L, Gokemeijer J, Shamah S, Warikoo V, et al. Antagonists to human and mouse vascular endothelial growth factor receptor 2 generated by directed protein evolution in vitro. Chem Biol 2006; 13: 549–56.
Silverman J, Liu Q, Bakker A, To W, Duguay A, Alba BM, et al. Multivalent avimer proteins evolved by exon shuffling of a family of human receptor domains. Nat Biotechnol 2005; 23: 1556–61.
Binz HK, Stumpp MT, Forrer P, Amstutz P, Plnckthun A . Designing repeat proteins: well-expressed, soluble and stable proteins from combinatorial libraries of consensus ankyrin repeat proteins. J Mol Biol 2003; 332: 489–503.
Zahnd C, Wyler E, Schwenk JM, Steiner D, Lawrence MC, McKern NM, et al. A designed ankyrin repeat protein evolved to picomolar affinity to Her2. J Mol Biol 2007; 369: 1015–28.
Binz HK, Amstutz P, Kohl A, Stumpp MT, Briand C, Forrer P, et al. High-affinity binders selected from designed ankyrin repeat protein libraries. Nat Biotechnol 2004; 22: 575–82.
Nygren PA . Alternative binding proteins: affibody binding proteins developed from a small three-helix bundle scaffold. FEBS J 2008; 275: 2668–76.
Schlehuber S, Skerra A . Lipocalins in drug discovery: from natural ligand-binding proteins to "anticalins". Drug Discov Today 2005; 10: 23–33.
Colas P, Cohen B, Jessen T, Grishina I, McCoy J, Brent R, et al. Genetic selection of peptide aptamers that recognize and inhibit cyclin-dependent kinase 2. Nature 1996; 380: 548–50.
Binz HK, Amstutz P, Pluckthun A . Engineering novel binding proteins from nonimmunoglobulin domains. Nat Biotechnol 2005; 23: 1257–68.
Chen JD, Yang Q, Yang AG, Marasco WA, Chen SY . Intra- and extracellular immunization against HIV-1 infection with lymphocytes transduced with an AAV vector expressing a human anti-gp120 antibody. Hum Gene Ther 1996; 7: 1515–25.
Tanaka T, Lobato MN, Rabbitts TH . Single domain intracellular antibodies: a minimal fragment for direct in vivo selection of antigen-specific Intrabodies. J Mol Biol 2003; 331: 1109–20.
Jendreyko N, Popkov M, Rader C, Barbas CF . Phenotypic knockout of VEGF-R2 and Tie-2 with an intradiabody reduces tumor growth and angiogenesis in vivo. Proc Natl Acad Sci USA 2005; 102: 8293–8.
Cohen PA . Intrabodies. Targeting scFv expression to eukaryotic intracellular compartments. Methods Mol Biol 2002; 178: 367–78.
Boldicke T, Weber H, Mueller PP, Barleon B, Bernal M . Novel highly efficient intrabody mediates complete inhibition of cell surface expression of the human vascular endothelial growth factor receptor-2 (VEGFR-2/KDR). J Immunol Methods 2005; 300: 146–59.
Jannot CB, Beerli RR, Mason S, Gullick WJ, Hynes NE . Intracellular expression of a single-chain antibody directed to the EGFR leads to growth inhibition of tumor cells. Oncogene 1996; 13: 275–82.
Shin I, Edl J, Biswas S, Lin PC, Mernaugh R, Arteaga CL, et al. Proapoptotic activity of cell-permeable anti-Akt single-chain antibodies. Cancer Res 2005; 65: 2815–24.
Paz K, Brennan LA, Iacolina M, Doody J, Hadari YR, Zhu Z, et al. Human single-domain neutralizing intrabodies directed against Etk kinase: a novel approach to impair cellular transformation. Mol Cancer Ther 2005; 4: 1801–9.
Strube RW, Chen SY . Characterization of anti-cyclin E single-chain Fv antibodies and intrabodies in breast cancer cells: enhanced intracellular stability of novel sFv-Fc intrabodies. J Immunol Methods 2002; 263: 149–67.
Williams BR, Zhu Z . Intrabody-based approaches to cancer therapy: status and prospects. Curr Med Chem 2006; 13: 1473–80.
Mhashilkar AM, LaVecchio J, Eberhardt B, Porter-Brooks J, Boisot S, Dove JH, et al. Inhibition of human immunodeficiency virus type 1 replication in vitro in acutely and persistently infected human CD4+ mononuclear cells expressing Murine and humanized anti-human immunodeficiency virus type 1 Tat single-chain variable fragment intrabodies. Human Gene Ther 1999; 10: 1453–67.
Steinberger P, Andris-Widhopf J, Bühler B, Torbett BE, Barbas CF . Functional deletion of the CCR5 receptor by intracellular immunization produces cells that are refractory to CCR5-dependent HIV-1 infection and cell fusion. Proc Natl Acad Sci USA 2000; 97: 805–10.
Doorbar J, Griffin H . Intrabody strategies for the treatment of human papillomavirus-associated disease. Expert Opin Biol Ther 2007; 7: 677–89.
Messer A, Lynch SM, Butler DC . Developing intrabodies for the therapeutic suppression of neurodegenerative pathology. Expert Opin Biol Ther 2009; 9: 1189–97.
Mhashilkar AM, Doebis C, Seifert M, Busch A, Zani C, Soo Hoo J, et al. Intrabody-mediated phenotypic knockout of major histocompatibility complex class I expression in human and monkey cell lines and in primary human keratinocytes. Gene Ther 2002; 9: 307–19.
Alvarez RD, Barnes MN, Gomez-Navarro J, Wang M, Strong TV, Arafat W, et al. A cancer gene therapy approach utilizing an anti-erbB-2 single-chain antibody-encoding adenovirus (AD21): a phase I trial. Clin Cancer Res 2000; 6: 3081–7.
Nakata Y, Kim TK, Shetzline S, Gewirtz AM . Nucleic acid modulation of gene expression: approaches for nucleic acid therapeutics against cancer. Crit Rev Eukaryot Gene Expr 2005; 15: 163–82.
Persengiev SP, Zhu X, Green MR . Nonspecific, concentration-dependent stimulation and repression of mammalian gene expression by small interfering RNAs (siRNAs). RNA 2004; 10: 12–8.
Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science 2003; 302: 415–9.
Nielsen UB, Kirpotin DB, Pickering EM, Hong K, Park JW, Refaat Shalaby M, et al. Therapeutic efficacy of anti-ErbB2 immunoliposomes targeted by a phage antibody selected for cellular endocytosis. Biochim Biophys Acta Mol Cell Res 2002; 1591: 109–18.
Sapra P, Moase EH, Ma J, Allen TM . Improved Therapeutic Responses in a Xenograft Model of Human B Lymphoma (Namalwa) for Liposomal Vincristine versus Liposomal Doxorubicin Targeted via Anti-CD19 IgG2a or Fab' fragments. Clin Cancer Res 2004; 10: 1100–11.
Elliott G, O'Hare P . Intercellular trafficking and protein delivery by a Herpesvirus structural protein. Cell 1997; 88: 223–33.
Frankel AD, Pabo CO . Cellular uptake of the tat protein from human immunodeficiency virus. Cell 1988; 55: 1189–93.
Tse E, Lobato MN, Forster A, Tanaka T, Chung GT, Rabbitts TH . Intracellular antibody capture technology: application to selection of intracellular antibodies recognising the BCR-ABL oncogenic protein. J Mol Biol 2002; 317: 85–94.
Gennari F, Mehta S, Wang Y, St Clair Tallarico A, Palu G, et al. Direct phage to intrabody screening (DPIS): demonstration by isolation of cytosolic intrabodies against the TES1 site of Epstein Barr virus latent membrane protein 1 (LMP1) that block NF-[kappa]B transactivation. J Mol Biol 2004; 335: 193–207.
der Maur AA, Zahnd C, Fischer F, Spinelli S, Honegger A, Cambillau C, et al. Direct in vivo screening of intrabody libraries constructed on a highly stable single-chain framework. J Biol Chem 2002; 277: 45075–85.
World's top-selling drugs in 2014 vs 2010. 2010. Reuters News. 4-13-2010. Ref Type: Report
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, J., Zhu, Z. Research and development of next generation of antibody-based therapeutics. Acta Pharmacol Sin 31, 1198–1207 (2010). https://doi.org/10.1038/aps.2010.120
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2010.120
Keywords
This article is cited by
-
Identification, characterization and control of a sequence variant in monoclonal antibody drug product: a case study
Scientific Reports (2021)
-
Recent developments in affinity-based selection of aptamers for binding disease-related protein targets
Chemical Papers (2019)
-
Impact of a Heat Shock Protein Impurity on the Immunogenicity of Biotherapeutic Monoclonal Antibodies
Pharmaceutical Research (2019)
-
On-line glucose monitoring by near infrared spectroscopy during the scale up steps of mammalian cell cultivation process development
Bioprocess and Biosystems Engineering (2019)
-
Evaluating the expression profile and stability of different UCOE containing vector combinations in mAb-producing CHO cells
BMC Biotechnology (2017)