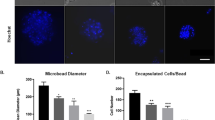
Abstract
We describe a technique for the treatment of malignant brain tumors based on local delivery of the anti-angiogenic protein endostatin from genetically engineered cells encapsulated in ultrapure sodium alginate. Alginate consists of L-guluronic and D-mannuronic acid, which in the presence of divalent cations forms an extended gel network, in which cells reside and remain immunoisolated, when implanted into the rat brain. Here, we show that endostatin-transfected cells encapsulated in alginate maintain endostatin secretion for at least four months after intracerebral implantation in rats. During the implantation period 70% of the encapsulated cells remained viable, as opposed to 85% in in vitro-cultured capsules. Rats that received transplants of BT4C glioma cells, together with endostatin-producing capsules (0.2 μg/ml per capsule), survived 84% longer than the controls. The endostatin released from the capsules led to an induction of apoptosis, hypoxia, and large necrotic avascular areas within 77% of the treated tumors, whereas all the controls were negative. The encapsulation technique may be used for many different cell lines engineered to potentially interfere with the complex microenvironment in which tumor and normal cells reside. The present work may thus provide the basis for new therapeutic approaches toward brain tumors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Scherer, H.J. Structural development in gliomas. Am. J. Cancer 34 , 333–351 (1938).
Plate, K.H. & Risau, W. Angiogenesis in malignant gliomas. Glia 15, 339–347 (1995).
Cao, R. et al. Suppression of angiogenesis and tumor growth by the inhibitor K1-5 generated by plasmin-mediated proteolysis. Proc. Natl. Acad. Sci. USA 96, 5728–5733 ( 1999).
Chung, J., Gao, A.G. & Frazier, W.A. Thrombospondin acts via integrin-associated protein to activate the platelet integrin alphaIIbbeta3. J. Biol. Chem. 272, 14740–14746 ( 1997).
O'Reilly, M.S. et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell 88, 277–285 (1997).
Chen. Q.R., Kumar, D., Stass, S.A. & Mixson, A.J. Liposomes complexed to plasmids encoding angiostatin and endostatin inhibit breast cancer in nude mice. Cancer Res. 59, 3308– 3312 (1999).
Dhanabal, M. et al. Endostatin: yeast production, mutants, and antitumor effect in renal cell carcinoma. Cancer Res. 59, 189–197 (1999).
Blezinger, P. et al. Systemic inhibition of tumor growth and tumor metastases by intramuscular administration of the endostatin gene. Nat. Biotechnol. 17, 343–348 ( 1999).
Kleihues, P., Burger, P.C., Plate, K.H., Ohgaki, H. & Cavanee, W.K. Glioblastoma. In Pathology and genetics of tumours of the central nervous system. (eds Kleihues, P. & Cavanee, W.K.) 16–24 (The International Agency for Research on Cancer, Lyons, France; 1997).
Olivi, A., DiMeco, F., Bohan, E. & Brem H. Developing new methods for the treatment of malignant brain tumours: local delivery of anti-neoplastic agents using biodegradable polymers. Forum (Genova) 10, 152–165 (2000).
Hottinger, A.F. & Aebischer. P. Treatment of diseases of the central nervous system using encapsulated cells. Adv. Tech. Stand. Neurosurg. 25, 3– 20 (1999).
Lang, M.S., Hovenkamp, E., Savelkoul, H.F.J., Knegt, P. & van Ewijk, W. Immunotherapy with monoclonal antibodies directed against the immunosuppressive domain of p15E inhibits tumour growth. Clin. Exp. Immunol. 102, 468–475 (1995).
Lim, F. & Sun, A.M. Microencapsulated islets as bioartificial endocrine pancreas. Science 210, 908– 910 (1980).
Martinsen, A., Skjåk-Bræk, G. & Smidsrød, O. Alginate as immobilization material: I. Correlation between chemical and physical properties of alginate gel beads. Biotechnol. Bioeng. 33, 79– 89 (1989).
Martinsen, A., Storrø, I. & Skjåk-Bræk, G. Alginate as immobilization material: III. Diffusional properties. Biotechnol. Bioeng. 39, 186–194 (1992).
Read, T-A. et al. Cells encapsulated in alginate: a potential system for delivery of recombinant proteins to malignant brain tumours. Int. J. Dev. Neurosci. 17, 653–663 ( 1999).
Krewson, C.E. & Saltzman, W.M. Transport and elimination of recombinant human NGF during long-term delivery to the brain. Brain Res. 15, 169–181 ( 1996).
Mahoney, M.J. & Saltzman, W.M. Millimeter-scale positioning of a nerve-growth-factor source and biological activity in the brain. Proc. Natl. Acad. Sci. USA 13, 4536 –4539 (1999).
Thorsen, F., Read, T-A., Lund-Johansen, M, Tyssnes, B.B. & Bjerkvig, R. Alginate encapsulated producer cells: a potential new approach to the treatment of malignant brain tumours. Cell Transplant. (December 2000).
Ross, C.J., Ralph, M. & Chang, P.L. Delivery of recombinant gene products to the central nervous system with nonautologous cells in alginate microcapsules. Hum. Gene Ther. 10, 49–59 ( 1999).
Boucher, Y., Salehi, H., Witwer, B., Harsh, G.R & Jain, R.K. Interstitial fluid pressure in intracranial tumours in patients and in rodents. Br. J. Cancer 75, 829–836 (1997).
Steen, R.G., Kromhout-Schiro, S. & Graham, M.M. Relationship of perfusion to edema in the 9L glioma . J. Neurooncol. 16, 81– 87 (1993).
Dhanabal, M. et al. Endostatin induces endothelial cell apoptosis. J. Biol. Chem. 274, 11721–11726 (1999).
Chang, Z., Choon, A. & Friedl, A. Endostatin binds to blood vessels in situ independent of heparan sulfate and does not compete for fibroblast growth factor-2 binding . Am. J. Pathol. 155, 71– 76 (1999).
Yamaguchi, N. et al. Endostatin inhibits VEGF-induced endothelial cell migration and tumour growth independently of zinc binding. EMBO J. 18, 4414–4423 (1999).
Yao, K.S., Clayton, M. & O'Dwyer, P. Apoptosis in human adenocarcinoma HT29 cells induced by exposure to hypoxia. J. Natl. Cancer Inst. 87, 117–122 (1995).
Tohma, Y. et al. Necrogenesis and Fas/APO-1 (CD95) expression in primary (de novo) and secondary glioblastomas. Neuropathol. Exp. Neurol. 57, 239–245 (1998).
Plate, K.H., Breier, G., Weich, H.A. & Risau, W. Vascular endothelial growth factor is a potential tumor angiogenesis factor in human gliomas in vivo. Nature 359, 845–848 (1992).
Goldbrunner, R.H., Bernstein, J.J. & Tonn, J.C. Cell-extracellular matrix interaction in glioma invasion. Acta Neurochir. (Wien) 141, 295–305 (1999).
Lichtenbeld, H.C., Ferarra, N., Jain, R.K. & Munn, L.L. Effect of local anti-VEGF antibody treatment on tumor microvessel permeability . Microvasc. Res. 57, 357– 362 (1999).
Laerum, O.D. & Rajewsky, M.F. Neoplastic transformation of fetal rat brain cells in culture after exposure to ethylnitrosourea in vivo. J. Natl. Cancer Inst. 55, 1177– 1187 (1975).
Blake, M.S., Johnston, K.H., Russell-Jones, G.J. & Gotschlich, E.C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal. Biochem. 136, 175–179 (1984).
Sasaki, T. et al. Structure, function and tissue forms of the C-terminal globular domain of collagen XVIII containing the angiogenesis inhibitor endostatin . EMBO J. 17, 4249–4256 (1998).
Takeoka, T., Shinohara, Y., Furumi, K. & Mori, K. Characteristic protein fractions of cerebrospinal fluid disc electrophoretic analysis. Brain Res. 29, 147– 150 (1980).
Visted, T. et al. acZ-neoR transfected glioma cells in syngeneic rats: growth pattern and characterization of the host immune response against cells transplanted inside and outside the CNS. Int. J. Cancer 15, 228–235 (2000).
Walker, G.R., Feather, K.D., Davis, P.D. & Hines, K.K. SuperSignalMT CL-HPR: a new enhanced chemiluminescent substrate for the development of horseradish peroxidase label in western blotting applications . J. NIH Res. 7, 76 ( 1995).
Druckrey, H. Genotypes and phenotypes of ten inbred strains of BD-rats. Arzneimittelforschung 21, 1274–1278 ( 1971).
Varia, M.A. et al. Pimonidazole: a novel hypoxia marker for complementary study of tumour hypoxia and cell proliferation in cervical carcinoma. Gynecol. Oncol. 71, 270–277 (1998).
Mahesparan, R. et al. Extracellular matrix-induced cell migration from glioblastoma specimens in vitro. Acta Neuropathol. 97, 231–239 (1999).
Acknowledgements
We thank the Norwegian Cancer Society, the National Gene Therapy Program, the Norwegian Research Counsel and Innovest, and the University of Bergen for financial support toward this study. Furthermore, we thank Bodil Hansen, Tove Drange Johannsen, and Tore Jacob Raa for excellent technical assistance. Finally we thank Dr. Rupert Timpl at the Max-Planck institute, Martinsried, Germany for supplying the endostatin antiserum.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Read, TA., Sorensen, D., Mahesparan, R. et al. Local endostatin treatment of gliomas administered by microencapsulated producer cells. Nat Biotechnol 19, 29–34 (2001). https://doi.org/10.1038/83471
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/83471
This article is cited by
-
Control of temperature dependence of microbial time–temperature integrator (TTI) by microencapsulation of lactic acid bacteria into microbeads with different proportions of alginate
Food Science and Biotechnology (2021)
-
Localized targeted antiangiogenic drug delivery for glioblastoma
Journal of Neuro-Oncology (2018)
-
Enhanced expression of Vastatin inhibits angiogenesis and prolongs survival in murine orthotopic glioblastoma model
BMC Cancer (2017)
-
Imidazolium-based polymer hydrogels with microdomains as carriers of hydrophobic molecules
Polymer Journal (2014)
-
A novel multilayer immunoisolating encapsulation system overcoming protrusion of cells
Scientific Reports (2014)