Abstract
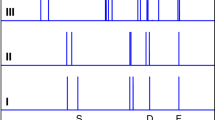
DESPITE its simplicity at the molecular level, water is a complex and poorly understood liquid1. The reasons for this centre around the existence of a dynamic hydrogen-bonded network throughout the liquid. Attempts to describe the structure of liquid water have tended to invoke either continuum models such as the 'distortedbond' model2, which assumes that the hydrogen-bonded structure relaxes on a timescale similar to that in other liquids, and mixture models, such as the 'flickering-cluster' model3, which postulate the coexistence of two or more long-lived structures in the liquid. Here we analyse inelastic neutron-scattering spectra for the ice I solid phase of water, which provide evidence for the existence of two different kinds of hydrogen-bond, of different strengths, in the solid. A model in which strong and weak hydrogen-bonds in the ratio of about 2:1 are randomly distributed throughout the network is able to reproduce the neutron spectra. If we can assume that the same kind of bimodal hydrogen-bonding exists in the liquid state, our model may be able to explain several of the anomalous properties of liquid water, such as the large specific heat and the unusual behaviour of water in thin films and clusters.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Frank, H. S. in Water: A Comprehensive Treatise Vol. 1 (ed. Franks, F.) 515–577 (Plenum, New York & London, 1972).
Pople, J. A. Proc. R. Soc. A205, 163–171 (1957).
Frank, H. S. & Wen, W.-Y., Discuss Farad. Soc. 24, 133–148 (1957).
Morokuma, K. & Pedersen, L. J. chem. Phys. 48, 3275–3282 (1968).
Millot, C. & Stone, A. J. Molec. Phys. 77, 439–462 (1992).
Lee, C. et al. Phys. Rev. Lett. 69, 462–465 (1992).
Finney, J. L., Quinn, J. E. & Baum, J. O. in Water Science Review Vol. 1 (ed. Franks, F.) 93–170 (Cambridge Univ. Press, 1985).
Whalley, E. & Bertie, J. E. J. chem. Phys. 46, 1271–1284 (1967).
Mineeva-Sukarova, B., Sherman, W. F. & Wilkinson, G. R. J. Phys. C17, 5833–5850 (1984).
Prask, H., Boutin, H. & Yip, S. J. chem. Phys. 48, 3367–3376 (1968).
Renker, B. in Physics and Chemistry of Ice (eds Whalley, E., Hones, S. J. & Gold, L. W.) 82–89 (Univ. of Toronto Press, 1973).
Chen, S.-H. Proc. of the NATO Adv. Study Inst. on Hydrogen-bonded Liquids (eds Dore, J. C. & Teixeira, J.) 289–295 (Kluwer Academic, London, 1991).
Sevensson, E. C. et al. in Phonon 89 (eds Hunklinger, S., Lugwig, W. & Weiss, G.) 537–541 (World Scientific, London, 1990).
Li, J-C., Ross, D. K., Hall, P. G. & Tomkinson, J. J. phys. B156/157, 376–379 (1989).
Li, J-C. et al. J. chem. phys. 94, 6770–6775 (1991).
Li, J-C. & Ross, D. K. Int. Conf. on Phys. and Chem. of Ice (eds Maeno, N. & Hondoh, T.) 27–34 (Hokkaido Univ. Press, 1992).
Li, J-C. et al. J. Phys. Condensed Matter 4, 2109–2116 (1992).
Marchi, M., Tse, J. S. & Klein, L. J. chem. Phys. 85, 2414–2419 (1986).
Kolesnikov, A. I. et al. Phys. Lett. A168, L308–L312 (1992).
Kistenmacher, H., Popkie, H. & Clementi, E. J. chem. Phys. 60, 4455–4465 (1972).
Stanley, H. E. & Teixeia, J. J. chem. Phys. 73, 3404–3422 (1980).
Li, J-C. et al. Inst. of Phys. Conf. Ser. Vol. 101 (eds Fairbanks, M. C, North, A. N. & Newport, R. J.) 109–118 (Arrowsmith, Bristol, 1990).
Etzler, F. M. & Drost-Hanson, W. Croatica Chemica Acta 56, 563–592 (1983).
Bernal, J. D. & Fowler, R. H. J. chem. Phys. 1, 515–548 (1933).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Li, J., Ross, D. Evidence for two kinds of hydrogen bond in ice. Nature 365, 327–329 (1993). https://doi.org/10.1038/365327a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/365327a0
This article is cited by
-
A volumetric study of ionic interactions of ammonium sulfate in water and aqueous DMF at different temperatures
Chemical Papers (2022)
-
The normal modes of lattice vibrations of ice XI
Scientific Reports (2016)
-
Hydrogen-bond potential for ice VIII-X phase transition
Scientific Reports (2016)
-
The hydrogen bond donor and acceptor ability of thioformic acid
Structural Chemistry (2011)
-
Molecular dynamics study on the structure I xenon hydrate
Chinese Science Bulletin (2005)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.