Abstract
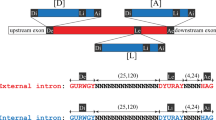
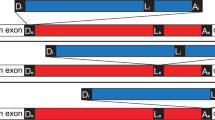
THE organization of eukaryotic genes into exons separated by introns has been considered as a primordial arrangement1,2 but because it does not exist in eubacterial genomes it may be that introns are relatively recent acquisitions3. A self-splicing group I intron has been found in cyanobacteria at the same position of the same gene (that encoding leucyl transfer RNA, UAA anticodon) as a similar group I intron of chloroplasts4,5, which indicates that this intron predates the invasion of eukaryotic cells by cyanobac-terial endosymbionts. But it is not clear from this isolated example whether introns are more generally present in different genes or in more diverse branches of the eubacteria. Many mitochondria have intron-rich genomes and were probably derived from the alpha subgroup of the purple bacteria6 (or Proteobacteria7), so ancient introns might also have been retained in these bacteria. We describe here the discovery of two small (237 and 205 nucleotides) self- splicing group I introns in members of two proteobacterial subgroups, Agrobacterium tumefaciens (α) and Azoarcus sp. (β). The introns are inserted in genes for tRNAArg and tRNAIle respectively, after the third anticodon nucleotide. Their occurrence in different genes of phyogenetically diverse bacteria indicates that group I introns have a widespread distribution among eubacteria.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Gilbert, W. Nature 271, 501 (1978).
Darnell, J. E. & Doolittle, W. F. Proc. natn. Acad. Sci. U.S.A. 83, 1271–1275 (1986).
Palmer, J. D. & Logsdon, J. M. Curr. Opin. Genet. Dev. 1, 470–477 (1991).
Kuhsel, M. G., Strickland, R. & Palmer, J. D. Science 250, 1570–1573 (1990).
Xu, M.-Q., Kathe, S. D., Goodrich-Blair, H., Nierzwicki-Bauer, S. A. & Shub, D. A. Science 250, 1566–1570 (1990).
Yang, D., Oyaizu, Y., Oyaizu, H., Olsen, G. J. & Woese, C. R. Proc. natn. Acad. Sci. U.S.A. 82, 4443–4447 (1985).
Stackebrandt, E., Murray, R. G. E. & Trüper, H. G. Int. J. syst. Bact. 38, 321–325 (1988).
Cech, T. R., Zaug, A. J. & Grabowski, P. J. Cell 27, 487–496 (1981).
Winans, S. C., Kerstetter, R. A. & Nester, E. W. J. Bact. 170, 4047–4054 (1988).
Kruger, K. et al. Cell 31, 147–157 (1982).
Cech, T. R. Gene 73, 259–271 (1988).
Michel, F. & Westhof, E. J. molec. Biol. 216, 585–610 (1990).
Antao, V. P., Lai, S. Y. & Tinoco, I. Jr Nucleic Acids Res. 19, 5901–5905 (1991).
Muramatsu, T. et al. Nature 336, 179–181 (1988).
Sprinzl, M., Dank, N., Nock, S. & Schön, A. Nucleic Acids Res. 19, (suppl.) 2127–2171 (1991).
Schulman, L. H. & Pelka, H. Science 242, 765–768 (1988).
Lee, C. P., Seong, B. L. & RajBhandary, U. L. J. biol. Chem. 266, 18012–18017 (1991).
Johnson, P. F. & Abelson, J. Nature 302, 681–687 (1983).
van Tol, H. & Beier, H. Nucleic Acids Res. 16, 1951–1966 (1988).
Choffat, Y., Suter, B., Behra, R. & Kubli, E. Molec. cell. Biol. 8, 3332–3337 (1988).
Nagel, G. M. & Doolittle, R. F. Proc. natn. Acad. Sci. U.S.A. 88, 8121–8125 (1991).
Woodson, S. A. & Cech, T. R. Cell 57, 335–345 (1989).
Woese, C. R. Microbiol. Rev. 51, 221–271 (1987).
Young, J. P. W. in Biological Nitrogen Fixation (eds Stacey, G., Burris, R. H. & Evans, H. J.) 43–86 (Chapman & Hall, New York, 1992).
Gott, J. M., Shub, D. A. & Belfort, M. Cell 47, 81–87 (1986).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Reinhold-Hurek, B., Shub, D. Self-splicing introns in tRNA genes of widely divergent bacteria. Nature 357, 173–176 (1992). https://doi.org/10.1038/357173a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/357173a0
This article is cited by
-
Multispecies autocatalytic RNA reaction networks in coacervates
Communications Chemistry (2023)
-
tRNA-m1A modification promotes T cell expansion via efficient MYC protein synthesis
Nature Immunology (2022)
-
Darwinian properties and their trade-offs in autocatalytic RNA reaction networks
Nature Communications (2021)
-
Negative Epistasis in Experimental RNA Fitness Landscapes
Journal of Molecular Evolution (2017)
-
Serial transfer can aid the evolution of autocatalytic sets
Journal of Systems Chemistry (2014)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.