Abstract
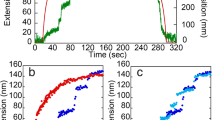
Extracellular matrix proteins are thought to provide a rigid mechanical anchor that supports and guides migrating and rolling cells1,2,3,4. Here we examine the mechanical properties of the extracellular matrix protein tenascin by using atomic-force-microscopy techniques. Our results indicate that tenascin is an elastic protein. Single molecules of tenascin could be stretched to several times their resting length. Force–extension curves showed a saw-tooth pattern, with peaks of force at 137 pN. These peaks were ∼25 nm apart. Similar results have been obtained by study of titin5. We also found similar results by studying recombinant tenascin fragments encompassing the 15 fibronectin type III domains of tenascin. This indicates that the extensibility of tenascin may be due to the stretch-induced unfolding of its fibronectin type III domains. Refolding of tenascin after stretching, observed when the force was reduced to near zero, showed a double-exponential recovery with time constants of 42 domains refolded per second and 0.5 domains per second. The former speed of refolding is more than twice as fast as any previously reported speed of refolding of a fibronectin type III domain6,7. We suggest that the extensibility of the modular fibronectin type III region may be important in allowing tenascin–ligand bonds to persist over long extensions. These properties of fibronectin type III modules may be of widespread use in extracellular proteins containing such domain8,9.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Palecek, S. P., Loftus, J. C., Ginsberg, M. H., Lauffenberger, D. A. & Horwitz, A. F. Integrin–ligand binding properties govern cell migration speed through cell–substratum adhesiveness. Nature 385, 537–540 (1997).
Lauffenburger, D. A. & Horwitz, A. F. Cell migration: a physically integrated molecular process. Cell 84, 359–369 (1996).
Alon, R., Hammer, D. A. & Springer, T. A. Lifetime of the P-selectin carbohydrate bond and its response to tensile force in hydrodynamic flow. Nature 374, 539–542 (1995).
Alon, R.et al. The kinetics of L-selectin tethers and the mechanics of selectin-mediated rolling. J. Cell Biol. 138, 1169–1180 (1997).
Rief, M., Gautel, M., Oesterhelt, F., Fernandez, J. M. & Gaub, H. E. Reversible unfolding of individual titin immunoglobin domains by AFM. Science 276, 1109–1112 (1997).
Plaxco, K. W.et al. Rapid refolding of a proline-rich all-β-sheet fibronectin type III module. Proc. Natl Acad. Sci. USA 93, 10703–10706 (1996).
Plaxco, K. W.et al. Acomparison of the folding kinetics and thermodynamics of two homologous fibronectin type III domains. J. Mol. Biol. 270, 763–770 (1997).
Bork, P.et al. Structure and distribution of modules in extracellular proteins. Quart. Rev. Biophys. 29, 119–167 (1996).
Campbell, I. D. & Spitzfaden, C. Building proteins with fibronectin type III modules. Structure 2, 333–337 (1994).
Erickson, H. P. Tenascin-C, tenascin-R and tenascin-X: a family of talented proteins in search of functions. Curr. Opin. Cell Biol. 5, 869–876 (1993).
Chiquet-Ehrismann, R. Tenascins, a growing family of extracellular matrix proteins. Experientia 51, 853–862 (1995).
Clark, R., Erickson, H. P. & Springer, T. A. Tenascin supports lymphocyte rolling. J. Cell Biol. 137, 755–765 (1997).
Bork, P. & Doolittle, R. F. Proposed acquisition of an animal protein domain by bacteria. Proc. Natl Acad. Sci. USA 89, 8990–8994 (1992).
Potts, J. R. & Campbell, I. D. Structure and function of fibronectin modules. Matrix Biol. 15, 313–320 (1996).
Keller, T. C. S. Molecular bungees. Nature 387, 233–235 (1997).
Tskhovrebova, L., Trinick, J., Sleep, J. A. & Simmons, R. M. Elasticity and unfolding of single molecules of the giant muscle protein titin. Nature 387, 308–312 (1997).
Kellermayer, S. B., Smith, S. B., Granzier, H. L. & Bustanente, C. Folding-unfolding transitions in single titin molecules characterized with lazer tweezers. Science 276, 1112–1116 (1997).
Rief, M.et al. Single molecule force spectroscopy on polysaccharides by atomic force microscopy. Science 275, 1295–1297 (1997).
Florin, E. L., Moy, V. T. & Gaub, H. E. Adhesion forces between individual ligand-receptor pairs. Science 264, 415–417 (1994).
Radmacher, M., Fritz, M., Hansma, H. G. & Hansma, P. K. Direct observation of enzyme activity with the atomic force microscope. Science 265, 1577–1579 (1994).
Aukhil, I.et al. Cell- and heparin-binding domains of the hexabrachion arm identified by tenascin expression proteins. J. Biol. Chem. 268, 2542–2552 (1993).
Marko, J. F. & Siggia, E. D. Stretching DNA. Macromolecules 28, 8759–8770 (1995).
Leahy, D. J., Hendrickson, W. A., Aukhil, I. & Erickson, H. P. Structure of a fibronectin type III domain from tenascin phased by MAD analysis of the selenomethionylprotein. Science 258, 987–991 (1992).
Clarke, J., Hamill, S. J. & Johnson, C. M. Folding and stability of a fibronectin type III domain of human tenascin. J. Mol. Biol. 270, 771–778 (1997).
Florin, E. L.et al. Sensing specific molecular interactions with the atomic force microscope. Biosens. Biolelectr. 10, 895–901 (1995).
Bell, G. I. Models for the specific adhesion of cells to cells. Science 200, 618–627 (1978).
Hammer, D. A. & Apte, S. M. Simulation of cell rolling and adhesion on surfaces in shear flow: general results and analysis of selectin-mediated neutrophil adhesion. Biophys. J. 63, 35–57 (1992).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oberhauser, A., Marszalek, P., Erickson, H. et al. The molecular elasticity of the extracellular matrix protein tenascin. Nature 393, 181–185 (1998). https://doi.org/10.1038/30270
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/30270
This article is cited by
-
Templated folding of the RTX domain of the bacterial toxin adenylate cyclase revealed by single molecule force spectroscopy
Nature Communications (2022)
-
The role of single-protein elasticity in mechanobiology
Nature Reviews Materials (2022)
-
Microbial production of megadalton titin yields fibers with advantageous mechanical properties
Nature Communications (2021)
-
Force spectroscopy of single cells using atomic force microscopy
Nature Reviews Methods Primers (2021)
-
Protein nanomechanics in biological context
Biophysical Reviews (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.