Abstract
Gemin5 is a 170-kDa WD-repeat-containing protein that was initially identified as a component of the survival of motor neurons (SMN) complex. We now show that Gemin5 facilitates the activation of apoptosis signal-regulating kinase 1 (ASK1) and downstream signaling. Gemin5 physically interacted with ASK1 as well as with the downstream kinases SEK1 and c-Jun NH2-terminal kinase (JNK1), and it potentiated the H2O2-induced activation of each of these kinases in intact cells. Moreover, Gemin5 promoted the binding of ASK1 to SEK1 and to JNK1, as well as the ASK1-induced activation of JNK1. In comparison, Gemin5 did not physically associate with MKK7, MKK3, MKK6, or p38. Furthermore, depletion of endogenous Gemin5 by RNA interference (RNAi) revealed that Gemin5 contributes to the activation of ASK1 and JNK1, and to apoptosis induced by H2O2 and tumor necrosis factor-α (TNFα) in HeLa cells. Together, our results suggest that Gemin5 functions as a scaffold protein for the ASK1–JNK1 signaling module and thereby potentiates ASK1-mediated signaling events.
Similar content being viewed by others
Main
Mitogen-activated protein kinase (MAPK) signaling pathways mediate the induction by diverse extracellular stimuli of various cellular activities, including cell growth, differentiation, and death.1, 2 MAPKs in mammalian cells include extracellular signal-regulated kinase (ERK), p38, and c-Jun NH2-terminal kinase (JNK; also known as stress-activated protein kinase or SAPK).1, 2 MAPK signaling pathways comprise modules of three kinases, including a MAPK kinase kinase (MAP3K), a MAPK kinase (MAP2K), and a MAPK.1 MAP3Ks phosphorylate and activate MAP2Ks, which in turn phosphorylate and activate MAPKs. Activated MAPKs phosphorylate various substrate proteins including transcription factors. Signaling by MAPK pathways is achieved either through a series of binary interactions between kinase components, or through the formation of a complex of multiple kinases mediated by a scaffold protein. There are several scaffold proteins that facilitate the activation of the MAPK signaling cascades. KSR and MP1 function as such scaffold proteins in the ERK signaling pathway,3, 4 whereas JNK-interacting protein 1 (JIP1), JNK/SAPK-associated protein 1 (JSAP1; also termed JIP3), and β-arrestin 2 do so in the JNK pathway.5, 6, 7, 8, 9, 10
The JNK signaling pathway is stimulated by exposure of cells to pro-inflammatory cytokines such as tumor necrosis factor-α (TNFα), or to cellular stresses such as genotoxic, osmotic, heat shock, hypoxic, and oxidative stresses.2 This pathway consists of JNK/SAPK, a MAP2K such as SEK1 (also known as MKK4 or JNKK1) or MKK7, and a MAP3K such as apoptosis signal-regulating kinase 1 (ASK1). ASK1 mediates the induction of apoptosis by a variety of intrinsic and extrinsic stresses. For instance, ASK1 is thought to participate in activation of the JNK pathway and apoptosis induced by withdrawal of nerve growth factor in sympathetic neurons as well as in seizure-induced neuronal death.11, 12 ASK1 is also implicated in other biological events, including the differentiation of various cell types.13, 14 Many proteins have been shown to bind ASK1, thereby positively or negatively regulating ASK1 signaling. Such proteins include thioredoxin, glutathione S-transferase (GST) mu, heat shock protein 72, p21, TRAF2, Daxx, and CIIA.13, 15, 16, 17, 18, 19, 20, 21
Gemin5 is a 170-kDa tryptophan–aspartic acid (WD)-repeat-containing protein that was initially identified as a component of the survival of motor neurons (SMN) complex.22, 23 The biological function of Gemin5 has remained unclear, however. Each WD repeat is composed of 40–60 amino acids with glycine–histidine and WD dipeptides at the amino- and C-terminal ends, respectively.24, 25 The β-propeller structure of WD-repeat domains underlies multiple protein–protein interactions,24 and proteins containing such domains are thought to perform diverse functions in many cellular processes, including signal transduction, vesicular trafficking, cell cycle regulation, and programmed cell death.25, 26
To provide insight into the biological function of Gemin5, we have now investigated the possible role of this protein in the MAPK signaling events. Our results show that Gemin5 promotes the ASK1-induced activation of JNK1 by functioning as a scaffold protein for the ASK1–JNK1 signaling module. Depletion of Gemin5 by RNA interference (RNAi) also reveals that this protein is a critical component in the activation of ASK1 and apoptosis induced by H2O2 or TNFα. These results suggest that Gemin5 is a natural potentiator of the ASK1–JNK1 signaling axis.
Results
Gemin5 physically interacts with ASK1
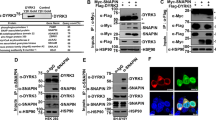
We initially searched for ASK1-binding proteins with using a yeast two-hybrid screen of an adult mouse brain cDNA library, and previously reported that GST mu and CIIA physically interact with ASK1 and thereby inhibit its kinase activity.19, 20 From the yeast two-hybrid screening, we also identified Gemin5 as another ASK1-binding protein. To examine further the physical interaction between Gemin5 and ASK1, we transfected 293T cells with expression vectors for HA-ASK1 and c-Myc epitope-tagged Gemin5 (Gemin5-Myc). Co-immunoprecipitation analysis showed that ASK1 physically associated with Gemin5 in the transfected cells (Figure 1a). We also examined whether endogenous ASK1 and Gemin5 proteins physically interact in intact cells. Immunoblot analysis with anti-ASK1 antibody of Gemin5 immunoprecipitates indeed revealed a physical association between the two endogenous proteins (Figure 1b). The extent of the physical interaction between ectopic ASK1 and Gemin5 in transfected 293T cells was altered by exposure of cells to H2O2, with the maximal increase at 30 min (Figure 1c). Subsequently, we observed that treatment of cells with H2O2 (1 mM for 30 min) or TNFα (20 ng/ml for 15 min) resulted in an increase in the interaction between endogenous ASK1 and Gemin5 proteins in 293T cells (Figure 1d). We next performed in vitro binding experiments to confirm the direct interaction between Gemin5 and ASK1. Incubation of in vitro-translated 35S-labeled Gemin5 with recombinant GST fusion proteins of ASK1 variants (ASK1-NT, -K, and -CT) revealed that Gemin5 directly bound to ASK1-NT and ASK1-K, which contains the kinase domain of ASK1 (Figure 1e). We also performed co-immunoprecipitation to examine the binding of Gemin5 to full-length ASK1, ASK1-NT, ASK1-K, and ASK1-ΔN in 293T cells that had been transfected with various combinations of plasmid vectors encoding the indicated proteins (Figure 1f). Gemin5 physically interacted with full-length ASK1 (amino acids 1–1375), ASK1-NT (amino acids 1–656), and ASK1-ΔN (amino acids 649–1375), but not with ASK1-K (amino acids 656–1001). The extent of Gemin5 binding to ASK1-NT or to ASK1-ΔN appears to be comparable to that of it for full-length ASK1. It is not clear why Gemin5 binding to ASK1-K was observed in vitro (Figure 1e) but not in co-immunoprecipitation experiments (Figure 1f). Nonetheless, these results suggest that ASK1 might have at least two independent binding sites for Gemin5.
Physical association of Gemin5 and ASK1 in intact cells. (a) Interaction between ectopic Gemin5 and ASK1 in transfected 293T cells. 293T cells were transfected for 48 h with a plasmid encoding HA-ASK1, and either a vector for Gemin5-Myc or the corresponding empty vector. Cell lysates were subjected to immunoprecipitation (IP) with antibody to HA, and the resulting precipitates were subjected to immunoblot (IB) analysis with antibody to Myc. Cell lysates were also subjected directly to immunoblot analysis with antibodies to Myc and to HA. IgGH, the heavy chain of immunoglobulin G. (b) Interaction between endogenous Gemin5 and ASK1 in 293T cells. Cell lysates were subjected to IP with mouse pre-immune IgG or antibody to Gemin5, and the resulting precipitates were subjected by IB analysis with antibody to ASK1. Cell lysates were also subjected directly to IB analysis with antibodies to ASK1 and to Gemin5. (c and d) Effect of H2O2 on the interaction between Gemin5 and ASK1 in intact cells. (c) 293T cells were transfected for 48 h with a plasmid encoding Gemin5-Myc, and either a vector for HA-ASK1 or the corresponding empty vector. The cells were then left untreated or treated with 1 mM H2O2 for the indicated times. Cell lysates were subjected to IP with anti-Myc antibody and the resulting precipitates were examined by IB analysis with anti-HA antibody. Cell lysates were also examined directly by IB analysis with antibodies to HA or to Myc. (d) 293T cells were incubated in the absence or presence of H2O2 (1 mM) for 30 min or TNFα (20 ng/ml) for 15 min. Cell lysates were treated with mouse monoclonal anti-Gemin5 antibody or mouse pre-immune IgG, and the resulting precipitates were subjected to IB analysis with anti-ASK1 antibody. Cell lysates were also subjected directly to IB analysis with antibodies to ASK1 or to Gemin5. (e) In vitro assay of the binding between Gemin5 and variants of ASK1. In vitro-translated 35S-labeled Gemin5 was incubated for 3 h at 4°C with GST fusion proteins (1 μg each) of ASK1-NT, ASK1-K, or ASK1-CT in a final volume of 500 μl. The GST fusion proteins were then precipitated with glutathione–agarose beads, and associated 35S-labeled Gemin5 was detected by SDS-PAGE and autoradiography (middle panel). A portion (2%) of the Gemin5 input to the binding mixture was also directly analyzed by SDS-PAGE and autoradiography. The polyacrylamide gel was also stained with Coomassie Blue R-250 (lower panel). Schematic representations of the deletion mutants of ASK1, with the kinase domain shaded, are shown in the upper panel. (f) Co-IP of Gemin5 and variants of ASK1. 293T cells were transfected for 48 h with expression vectors encoding HA-tagged forms of ASK1, ASK1ΔN, ASK1-NT, and ASK1-K, in the absence or presence of a vector for Gemin5-Myc. Cell lysates were then subjected to immunoprecipitation with anti-Myc antibody, and the resulting precipitates were subjected to IB analysis with anti-HA antibody. Cell lysates (1% of the input for IP) were also subjected directly to IB analysis with antibodies to HA or to Myc. IgGL, the light chain of IgG
Gemin5 potentiates the H2O2-induced activation of ASK1, SEK1, and JNK1
We next examined whether Gemin5 affects ASK1 activity with using 293T cells expressing HA-ASK1 alone or HA-ASK1 plus Gemin5-V5. Exposure of the transfected cells to H2O2 resulted in an increase in ASK1 activity, but this increase was more pronounced in those expressing Gemin5-V5 (Figure 2a). The H2O2-stimulated activities of SEK1 and JNK1 were also potentiated by ectopic Gemin5 in transfected 293T cells (Figure 2b and c). In comparison, Gemin5 did not affect the H2O2-stimulated activity of p38 (data not shown). In contrast to its effect on ASK1 activity, ectopic Gemin5 did not affect the activation of MLK3, another MAP3K in the JNK signaling pathway,27 induced by ultraviolet (UV) irradiation (Figure 2d). Gemin5 also had no effect on the activation of ERK2 induced by phorbol-12-myristate 13-acetate (Figure 2e).
Gemin5 potentiates the activation of the ASK1–SEK1–JNK1 pathway but not the ERK2 pathway. (a–c) Effects of Gemin5 on H2O2-induced activation of ASK1, SEK1, and JNK1. 293T cells were transfected for 48 h with an expression vector for HA-ASK1 (a), Flag-SEK1 (b), or HA-JNK1 (c), alone or together with a vector for Gemin5-V5. The cells were then incubated for 20 min in the absence or presence of 1 mM H2O2, after which cell lysates were subjected to immunoprecipitation with antibodies to HA (a and c) or to Flag (b). The resulting precipitates were assayed for kinase activity of ASK1 (a), SEK1 (b), or JNK1 (c). Cell lysates were also subjected directly to immunoblot analysis with the indicated antibodies. The fold increase in each kinase activity relative to that of control cells is shown. (d) Gemin5 does not affect MLK3 activation induced by UV irradiation. 293T cells were transfected for 48 h with a vector encoding HA-MLK3, alone or together with a vector for Gemin5-V5. The cells were then exposed or not to UV light (60 J/m2) and incubated for an additional 30 min. Cell lysates were subjected to immunoprecipitation with an anti-HA antibody, and the resulting precipitates were assayed for MLK3 activity by immune complex kinase assay. Cell lysates were also subjected to immunoblot analysis with antibodies to HA and to V5. (e) Gemin5 does not affect ERK2 activation induced by phorbol-12-myristate-13-acetate (PMA). 293T cells were transfected for 40 h with a vector for HA-ERK2, alone or together with a vector for Gemin5-V5. The cells were then deprived of serum for 12 h and then untreated or treated for 15 min with 100 nM PMA. Cell lysates were subjected to immunoprecipitation with anti-HA antibody, and the resulting precipitates were assayed for ERK2 activity with MBP as substrate. Cell lysates were also subjected to immunoblot analysis with antibodies to HA and V5
Gemin5 potentiates the homo-oligomerization of ASK1 and its interaction with SEK1
Homo-oligomerization of ASK1 represents one mechanism of ASK1 activation.16, 18, 28, 29 We therefore examined whether Gemin5 affects ASK1 homo-oligomerization. 293T cells were transfected with vectors encoding HA-ASK1 and ASK1-Flag in the absence or presence of a vector for Gemin5-Myc. Co-immunoprecipitation analysis showed that HA-ASK1 physically associated with ASK1-Flag in the transfected cells, and that this homo-oligomerization of ASK1 was potentiated by coexpression of Gemin5 (Figure 3a). Gemin5 also enhanced the binding of ASK1 to its substrate SEK1 in transfected cells (Figure 3b). These results suggest that Gemin5 increases ASK1 activity, at least in part, by promoting both ASK1 homo-oligomerization and the binding of ASK1 to its substrate.
Gemin5 promotes ASK1 homo-oligomerization and the interaction between ASK1 and SEK1. (a) Effect of Gemin5 on ASK1 homo-oligomerization. 293T cells were transfected for 48 h with the indicated combinations of expression vectors encoding ASK1-Flag, HA-ASK1, and Gemin5-Myc. Cell lysates were subjected to immunoprecipitation with anti-HA antibody, and the resulting immunoprecipitates were subjected to immunoblot analysis with anti-Flag antibody. Cell lysates were also directly subjected to immunoblot analysis with antibodies to Myc, Flag, and HA. (b) Effect of Gemin5 on the interaction between ASK1 and SEK1. 293T cells were transfected for 48 h with the indicated combinations of vectors for Gemin5-V5, ASK1-Myc, and Flag-SEK1. Cell lysates were subjected to immunoprecipitation with anti-Myc antibody, and the resulting precipitates were subjected to immunoblot analysis with anti-Flag antibody. Cell lysates were also examined directly by immunoblot analysis with antibodies to Myc, V5, and Flag. IgGH, the heavy chain of IgG
Gemin5 functions as a scaffold to facilitate ASK1-induced activation of JNK1
Gemin5 contains up to 13 WD repeats.22 The β-propeller structures of WD repeats are thought to mediate multiple protein–protein interactions.24 Given that Gemin5 enhanced the physical interaction between ASK1 and SEK1 (Figure 3b), we investigated whether Gemin5 serves as a platform for the interactions among ASK1 and downstream components of the JNK signaling pathway, thereby facilitating activation of this pathway. Co-immunoprecipitation analysis revealed that Gemin5 physically associated with JNK1 (Figure 4a), as well as with SEK1 (Figure 4b), in transfected 293T cells. Interestingly, Gemin5 did not bind to MKK7 (Figure 4c), which is another MAP2K of the JNK signaling pathway.30 Gemin5 also had no effect on MKK7 activity stimulated by H2O2 in transfected 293T cells (data not shown). Physical association of endogenous Gemin5 with endogenous JNK1 and SEK1 was also confirmed (Figure 4d). Next, in order to examine which regions of Gemin5 were important for the interaction with ASK1, SEK1, or JNK1, we transfected 293T cells with expression vectors for three Gemin5 variants (Gemin5-WD, -Cen, and -Coil) and vectors for ASK1, SEK1, or JNK1, respectively. Co-immunoprecipitation data indicated that ASK1 physically associated with Gemin5-WD, Gemin5-Cen, and Gemin5-Coil in the transfected cells (Figure 4e). In comparison, SEK1 interacted with Gemin5-WD only, whereas JNK1 interacted with Gemin5-WD and Gemin5-Cen but not with Gemin5-Coil. To test the direct binding of Gemin5 to JNK1 and SEK1, we performed in vitro binding experiments. Incubation of in vitro-translated 35S-labeled Gemin5 with recombinant GST fusion proteins of JNK1, SEK, and p38 revealed that Gemin5 directly bound to JNK1 and SEK1, but not to p38 (Figure 4f). Co-immunoprecipitation data also indicated that Gemin5 did not physically associate with p38 (data not shown). Intriguingly, ectopic Gemin5 enhanced the physical interaction between ASK1 and JNK1 (Figure 5a), as well as ASK1-induced JNK1 activation in cotransfected 293T cells (Figure 5b). In contrast, Gemin5 did not affect the MLK3-induced activation of JNK1 (Figure 5c). Furthermore, Gemin5-Cen, which did not bind SEK1 (Figure 4e), failed to enhance ASK1-induced JNK1 activation (Figure 5d). Gemin5-Coil, which did not bind SEK1 or JNK1 (Figure 4e), also did not promote ASK1-induced JNK1 activation (Figure 5e). Taken together, these results suggest that full-length Gemin5 functions as a scaffold protein that facilitates activation of the ASK1–SEK1–JNK1 signaling pathway.
Gemin5 physically interacts with SEK1 and JNK1. (a–c) Physical association of Gemin5 with JNK1 and SEK1. 293T cells were transfected for 48 h with vectors encoding Gemin5-Myc and either HA-JNK1 (a) or Flag-SEK1 (b), or Flag-MKK7 (c), as indicated. Cell lysates were subjected to immunoprecipitation with antibodies to HA (a) or Flag (b and c), and the resulting precipitates were subjected to immunoblot analysis with antibody to Myc. Cell lysates were also directly examined by immunoblot analysis with the indicated antibodies. (d) Physical association of endogenous Gemin5 with endogenous JNK1 or SEK1 in 293T cells. 293T cells were subjected to immunoprecipitation with antibodies to JNK1 or SEK1, as indicated, and the resulting precipitates were immunoblotted with antibody to Gemin5. (e) Co-immunoprecipitation of Gemin5 variants and each of ASK1, SEK1, or JNK1 in transfected 293T cells. Cells were transfected for 48 h with expression vectors encoding Flag-tagged forms of Gemin5-WD, Gemin5-Cen, or Gemin5-Coil, in the absence or presence of a vector for ASK1-Myc, GST-SEK1, or JNK1-Myc, respectively. Cell lysates were then subjected to immunoprecipitation with the indicated antibodies, and the resulting precipitates were subjected to immunoblot analysis with anti-Flag antibody. Cell lysates (1% of the input for immunoprecipitation) were also subjected directly to immunoblot analysis with antibodies to Flag or to Myc or to GST. IgGH, the heavy chain of IgG. Schematic representations of the deletion mutants of human Gemin5 are shown in the left upper panel, with the WD40 repeats indicated by gray ovals and the coiled-coil motif marked with a shaded box. (f) In vitro binding of Gemin5 to JNK1 and SEK1. In vitro-translated 35S-labeled Gemin5 was incubated for 3 h at 4°C with GST fusion proteins (1 μg each) of JNK1, SEK1, and p38. The binding mixture was then applied to glutathione–agarose beads. Bead-bound proteins were eluted from the beads and associated 35S-labeled Gemin5 was detected by SDS-PAGE and autoradiography (upper panel). A portion (2%) of the 35S-labeled Gemin5 input to the binding reaction was also directly analyzed by SDS-PAGE and autoradiography. The polyacrylamide gel was also stained with Coomassie Blue R-250 (lower panel)
Gemin5 functions as a scaffold protein to potentiate ASK1-induced activation of JNK1. (a) Effect of Gemin5 on the interaction between ASK1 and JNK1. 293T cells were transfected for 48 h with the indicated combinations of expression vectors encoding ASK1-Myc, Flag-JNK1, and Gemin5-V5. Cell lysates were subjected to immunoprecipitation with anti-Flag antibody and the resulting precipitates were subjected to immunoblot analysis with anti-Myc antibody. Cell lysates were also directly examined by immunoblot analysis with the indicated antibodies. (b) Effect of Gemin5 on ASK1-mediated JNK1 activation. 293T cells were transfected for 48 h with an expression vector for HA-JNK1, alone or together with the indicated combinations of vectors for ASK1-Flag and Gemin5-V5. Cell lysates were subjected to immunoprecipitation with anti-HA antibody and the resulting precipitates were assayed for JNK1 activity by immune complex kinase assay. Cell lysates were also examined by immunoblot analysis with antibodies to HA, V5, and Flag. (c) Gemin5 does not affect the MLK3-induced activation of JNK1. 293T cells were transfected for 48 h with a vector encoding HA-JNK1, alone or together with vectors for MLK3 or Gemin5-V5. Cell lysates were subjected to immunoprecipitation with an antibody to HA, and the resulting precipitates were assayed for JNK1 activity. Cell lysates were also examined by immunoblot analysis with antibodies to HA, MLK3, or V5. (d and e) Effects of Gemin5-Cen or Gemin5-Coil on ASK1-mediated JNK1 activation. 293T cells were transfected for 48 h with an expression vector for HA-JNK1, alone or together with the indicated combinations of vectors for ASK1-Myc and Flag-tagged Gemin5-Cen or Gemin5-Coil. Cell lysates were subjected to immunoprecipitation with anti-HA antibody, and the resulting precipitates were assayed for JNK1 activity by immune complex kinase assay. Cell lysates were also examined by immunoblot analysis with antibodies to HA, Myc, or Flag
Given that the WD-repeat region was required for the binding of Gemin5 to SEK1 (Figure 4e), Gemin5 lacking the WD-repeat domain should not interact with SEK1. Indeed, when 293T cells were transfected with a vector for Gemin5 lacking the WD-repeat domain (Gemin5-ΔWD), and a vector for ASK1-Myc, GST-SEK1, or JNK1-Myc, co-immunoprecipitation analysis revealed that Gemin5-ΔWD physically associated with ASK1, but not with SEK1, in the transfected cells (Figure 6a and b). Gemin5-ΔWD also exhibited a weak interaction with JNK1 in the cells (Figure 6c). If the scaffold function is critical for Gemin5 to promote the activation of the ASK1–SEK1–JNK1 signaling axis, a Gemin5 mutant lacking any of the ASK1-, SEK1-, or JNK1-binding regions should not potentiate the activation of this signaling pathway. We, therefore, tested this possibility by examining the effect of Gemin5-ΔWD on the stimulation of ASK1, SEK1, and JNK1 activities induced by H2O2. Gemin5-ΔWD potentiated the H2O2-induced activation of ASK1, but failed to potentiate the H2O2-induced activation of SEK1 and JNK1 (Figure 6d–f). In fact, Gemin5-ΔWD, when overexpressed in higher levels in the transfected cells, blocked the activation of SEK1 and JNK1 activities induced by H2O2 (data not shown). In contrast, full-length Gemin5 facilitated the H2O2-induced activation of ASK1, SEK1, and JNK1 activities (Figure 2).
Gemin5-ΔWD does not interact with SEK1 and promote the activation of SEK1 and JNK1 induced by H2O2. (a–c) 293T cells were transfected for 48 h with a vector for Flag-Gemin5-ΔWD and a vector for ASK1-Myc (a), GST-SEK1 (b) or JNK1-Myc (c), as indicated. Cell lysates were subjected to immunoprecipitation with anti-Myc (a and c) or anti-GST antibodies (b), and the resulting precipitates were subjected to immunoblot analysis with an anti-Flag antibody. Cell lysates were also directly examined by immunoblot analysis with the indicated antibodies. (d–f) Effects of Gemin5-ΔWD on the H2O2-induced activation of ASK1, SEK1, and JNK1. 293T cells were transfected for 48 h with an expression vector for HA-ASK1 (d), GST-SEK1 (e), or HA-JNK1 (f), alone or together with a vector for Flag-Gemin5-ΔWD. The cells were then incubated for 20 min in the absence or presence of 1 mM H2O2, after which cell lysates were subjected to immunoprecipitation with antibodies to HA (d and f) or to GST (e). The resulting precipitates were assayed for kinase activities of ASK1 (d), SEK1 (e), or JNK1 (f). Cell lysates were also subjected directly to immunoblot analysis with the indicated antibodies
Knockdown of endogenous Gemin5 inhibits activation of ASK1 and JNK1, as well as apoptosis induced by H2O2 or TNFα in HeLa cells
To examine the role of endogenous Gemin5 in regulation of ASK1–JNK1 signaling, we transfected HeLa cells with vectors for two different Gemin5 small interfering RNAs (siRNAs) (named G5-siRNA1 and G5-siRNA2, respectively) and confirmed the depletion of Gemin5 expression in the Gemin5 siRNA-transfected cells by immunoblot analysis (Figure 7a). ASK1 has been shown to mediate the JNK signaling events induced by H2O2 and TNFα.31 The knockdown of Gemin5 expression by either G5-siRNA1 or G5-siRNA2 resulted in inhibition of the H2O2-induced activation of endogenous ASK1 and JNK1, compared with that apparent in cells transfected with a vector for a control siRNA (Figure 7a). Furthermore, the potentiating effect of Gemin5 on the H2O2-induced activation of endogenous ASK1 and JNK1 was rescued in G5-siRNA1-transfected cells by expression of the Gemin5 gene that contained a silent third-codon point mutation in the region targeted by G5-siRNA1 (Figure 7b). The G5-siRNA1-mediated knockdown of Gemin5 expression also inhibited the TNFα-induced activation of ASK1 and JNK1, compared with that of the control cells (Figure 7c). In comparison, the depletion of Gemin5 by RNAi did not affect the UV-induced activation of JNK1 (Figure 7d). The siRNA-mediated depletion of Gemin5 expression also inhibited the induction of apoptosis by H2O2 and TNFα, as assessed by DAPI staining (Figure 8a), by terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay (data not shown), and by Annexin V-FITC staining, followed by flow cytometry (Figure 8b). These results thus suggest that endogenous Gemin5 is critical for the promotion of ASK1-mediated JNK1 signaling in H2O2- and TNFα-induced signaling events.
siRNA-mediated knockdown of Gemin5 inhibits the H2O2- and TNFα-induced stimulation of ASK1 and JNK1 activities in HeLa cells. HeLa cells were stably transfected with vectors for GFP, Gemin5 siRNA1, or Gemin5 siRNA2, yielding HeLa/control (control), HeLa/Gemin5-siRNA1 (G5-siRNA1), or HeLa/Gemin5-siRNA2 (G5-siRNA2) cells, respectively. (a) HeLa/control, HeLa/G5-siRNA1, and HeLa/G5-siRNA2 cells were incubated in the absence or presence of 1 mM H2O2 for 20 min. Cell lysates were subjected to immunoprecipitation with antibodies to ASK1 or to JNK1, as indicated, and the resulting precipitates were assayed for ASK1 or JNK1 activity by immune complex kinase assay. (b) HeLa/Gemin5-siRNA1 cells were stably transfected with a vector for V5-tagged Gemin5 (Gemin5*-V5) encoded by the human Gemin5 gene possessing a silent point mutation in the region targeted by G5-siRNA1, and the stable transfectants were selected with G418 (500 μg/ml). HeLa/control, HeLa/G5-siRNA1, or HeLa/(G5-siRNA1+Gemin5*-V5) cells were incubated in the absence or presence of 1 mM H2O2 for 20 min. Cell lysates were examined for ASK1 or JNK1 activity by immune complex kinase assay as in (a). (c–d) HeLa/control or HeLa/G5-siRNA1 cells were incubated in the absence or presence of either 20 ng/ml TNFα for 20 min (c) or 60 J/m2 UV light (d). When irradiated with UV light, the cells were incubated further for 30 min. Cell lysates were examined for ASK1 or JNK1 activity by immune complex kinase assay as in (a). Cell lysates were also subjected directly to immunoblot analysis with antibodies to ASK1, JNK1, Gemin5, α-tubulin, or V5, as indicated
Depletion of Gemin5 expression by RNAi inhibits the H2O2- and TNFα-induced apoptosis in HeLa cells. (a) HeLa/control or HeLa/G5-siRNA1 cells were incubated for 24 h in the absence or presence of either H2O2 (0.8 mM) or TNFα (2.5 ng/ml) plus actinomycin D (0.1 μg/ml), after which the cells were analyzed for apoptosis by DAPI staining. The data of DAPI staining represent results from three independent experiments. Data are the mean of triplicate determinations±S.D. (b) HeLa/control or HeLa/G5-siRNA1 cells were incubated for 12 h in the absence or presence of either H2O2 (0.8 mM) or TNFα (2.5 ng/ml) plus actinomycin D (0.1 μg/ml). Cells were stained with Annexin V-FITC and PI and then analyzed for apoptotic cells (Annexin V-positive and PI-negative) by flow cytometry. Data are the mean of triplicate determinations±S.D. (a and b) *P<0.01, **P<0.01 for HeLa/control cells versus HeLa/G5-siRNA1 cells
Discussion
We have shown that Gemin5, a WD40-repeat protein, physically associates with ASK1 and potentiates the H2O2-induced activation of ASK1 and of ASK1-mediated downstream signaling events. Our results also revealed that Gemin5 promotes homo-oligomerization of ASK1, which represents one mechanism of ASK1 activation.16, 18, 28, 29 Moreover, Gemin5 enhances the physical interaction between ASK1 and SEK1. On the basis of these findings, we propose that Gemin5 potentiates ASK1-mediated signaling through at least two mechanisms: by promoting ASK1 homo-oligomerization and by facilitating the binding of ASK1 to its substrate.
Our binding studies showed that Gemin5 binds at least two distinct regions of ASK1. Interestingly, it also interacted with SEK1 and JNK. Furthermore, Gemin5 promoted the physical interaction between ASK1 and JNK1 in transfected cells under basal conditions, as well as potentiated the ASK1-induced activation of JNK1. These results suggest that Gemin5, like β-arrestin 2 and JSAP1/JIP3,8, 10 functions as a scaffold protein for the ASK1-dependent JNK signaling pathway. Gemin5 did not affect the MLK3-induced activation of JNK1, nor did it bind either to MKK7, another MAP2K for JNK1, or to MKK3 or MKK6 (data not shown), the MAP2Ks for p38 MAPK, or p38 MAPK. Our findings thus suggest that Gemin5 serves as a scaffold protein for the ASK1–SEK1–JNK1 signaling module. Unlike JSAP1/JIP3, the scaffold function of which is regulated by ASK1-dependent phosphorylation,10 Gemin5 was not phosphorylated by ASK1 in vitro (data not shown).
Gemin5 was originally identified as a component of the SMN complex,22 which plays an important role in the assembly of small nuclear ribonucleoproteins.32, 33 Deletion of the SMN gene causes spinal muscular atrophy (SMA), an autosomal recessive neuromuscular degeneration disease.23, 34, 35 Although Gemin5 is present in the SMN complex in gems, a nuclear structure similar to Cajal body, it is also localized throughout the cytoplasm.22, 36 The biological function of Gemin5, especially that of the protein localized in the cytoplasm, has remained obscure, however. Our results now suggest that Gemin5 facilitates the activation of the ASK1-dependent JNK1 signaling module, by forming a signaling complex with ASK1, SEK1, and JNK1. Knockdown of Gemin5 by siRNA confirmed that the endogenous protein contributes to the activation of ASK1 and JNK1, as well as apoptosis, by H2O2 or TNFα in HeLa cells. Potentiation by Gemin5 of ASK1-mediated signaling may be important to understand a biological function of Gemin5 in the SMN complex, as well as that in general. The possible relation between the function of the SMN complex and the promotion by Gemin5 of the activation of the ASK1–JNK1 signaling module remains to be studied in detail.
Materials and Methods
Plasmids, antibodies, and reagents
An expression vector for V5-tagged human Gemin5 (pcDNA3.1D/V5-His-TOPO-gemin5) was described previously.22 To generate an expression vector for Gemin5 tagged with the Myc epitope at its COOH-terminus, we amplified Gemin5 cDNA by PCR, using Myc-gemin5-1 (5′-ATTGGTACCATGGGGCAGGAGCCGCGG-3′ (KpnI site underlined)) and Myc-gemin5-2 (5′-ATTGCGGCCGCCCATACAGAAGGTCTG-3′ (NotI site underlined)) primers. The PCR product was then digested with KpnI and NotI and inserted into the corresponding sites of the pcDNA6/Myc-His B vector (Invitrogen). Expression vectors for hemagglutinin (HA) epitope-tagged ASK1 (HA-ASK1), Flag epitope-tagged ASK1 (ASK1-Flag), Myc epitope-tagged ASK1 (ASK1-Myc), Flag-SEK1, HA-JNK1, Flag-JNK1, and HA-ERK2 were described previously.19, 20, 37 Mouse monoclonal antibodies to HA, Flag, Myc, and V5 were purchased from Roche Applied Science, Sigma, Cell Signaling, and Invitrogen, respectively. Rabbit polyclonal antibodies to ASK1 and GST were from Santa Cruz biotechnology. Mouse monoclonal antibody to JNK1 was from BD Biosciences. Mouse monoclonal antibody to Gemin5 was described previously.22
Construction of Gemin5 deletion mutants
To construct mammalian expression vectors encoding deletion mutants of human Gemin5, we amplified the nucleotide sequences corresponding to the WD repeats (Gemin5-WD; amino acids 1–730), a central fragment (Gemin5-Cen; amino acids 731–1285), the coiled-coil domain (Gemin5-Coil; amino acids 1286–1508), and Gemin5 lacking the WD repeats (Gemin5-ΔWD; amino acids 731–1508) by PCR. Each PCR product was digested with NotI and KpnI and then inserted into the corresponding sites of p3 × FLAG-CMV-10 (Sigma) to generate p3 × FLAG-CMV-10/Gemin5-WD (encoding Flag-Gemin5-WD), p3 × FLAG-CMV-10/Gemin5-Cen (encoding Flag-Gemin5-Cen), p3 × FLAG-CMV-10/Gemin5-Coil (encoding Flag-Gemin5-Coil), and p3 × FLAG-CMV-10/Gemin5-ΔWD (encoding Flag-Gemin5-ΔWD). The PCR primers were 5′-ATTGCGGCCGCGATGGGGCAGGAGCCG-3′ and 5′-GCCGGTACCTCACTCCAATTCAATACT-3′ for Flag-Gemin5-WD; 5′-ATTGCGGCCGCGGAGAAAAAACGGCTC-3′ and 5′-ATTGGTACCTCACAGACGCCCATAAAG-3′ for Flag-Gemin5-Cen; 5′-ATTGCGGCCGCGTATGAATTCTGGTGG-3′ and 5′-ATTGGTACCTCACATACAGAAGGTCTG-3′ for Flag-Gemin5-Coil; 5′-ATTGCGGCCGCGGAGAAAAAACGGCTC-3′ and 5′-ATTGGTACCTCACATACAGAAGGTCTG-3′ for Flag-Gemin5-ΔWD (NotI and KpnI sites are underlined).
Construction of GST-fused ASK1 deletion mutants
For bacterial expression of GST fusion proteins of ASK1-NT, ASK1-K, and ASK1-CT, the nucleotide sequences for the ASK1 variants were amplified by PCR and subcloned into pGEX4T vectors (Amersham Biosciences). pGEX4T-1/ASK1-NT (encoding amino acids 1–656) was previously described.20 The PCR primers for ASK1-K were 5′-AGGGAATTCGAGAAGGGGAGAAGCACA-3′ (EcoRI site underlined) and 5′-AGGCTCGAGTTATGTTTTGAAAGAGAAGGG-3′ (XhoI site underlined). The PCR product was digested with EcoRI and XhoI and inserted into the corresponding sites of pGEX4T-1 to construct pGEX4T-1/ASK1-K (encoding amino acids 656–1001). The PCR primers for ASK1-CT were 5′-AGGGAATTCATTAAAATCTTCATGGAG-3′ and 5′-AGGCTCGAGTCAAGTCTGTTTGTTTCG-3′ (XhoI site underlined). The PCR product (1875 bp) was digested with EcoRI and XhoI, and then the internal EcoRI site-digested fragment (1086 bp) was isolated and inserted into the corresponding sites of pGEX4T-2 to construct pGEX4T-2/ASK1-CT (encoding amino acids 1014–1375).
Cell culture and DNA transfection
293T and HeLa cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (HyClone), in a humidified atmosphere of 5% CO2 at 37°C. For DNA transfection, 293T or HeLa cells were plated at 2 × 106 cells per 100-mm culture dish and transfected a day later with the indicated vectors, either by the calcium phosphate method or using Lipofectamine (Invitrogen).
Co-immunoprecipitation
Co-immunoprecipitation analysis was performed as previously described,20 with slight modifications. Cells were lysed in NETN buffer (0.5% Nonidet P-40 (NP-40), 1 mM EDTA, 50 mM Tris-HCl, pH 8.0, 120 mM NaCl) supplemented with 1 mM phenylmethylsulfonyl fluoride, 2 μg/ml leupeptin, 2 μg/ml aprotinin, and 2 mM Na3VO4. Cell lysates were subjected to centrifugation at 12 000 × g for 15 min at 4°C, and the resulting supernatants were incubated at 4°C, first for 2 h with appropriate antibodies, and then additionally for 1 h in the presence of protein G-conjugated Sepharose beads (Amersham Biosciences). The incubation mixtures were subjected to centrifugation at 12 000 × g for 15 min at 4°C, and the resulting immunoprecipitates were washed three times with NETN buffer and resolved by SDS-polyacrylamide gel electrophoresis (PAGE). The separated proteins were transferred electronically to an Immobilon-P transfer membrane (Millipore), which was then blocked with 5% non-fat dry milk before incubation for 1 h at room temperature with the indicated primary antibodies. Immunoreactive bands were visualized with horseradish peroxidase-conjugated secondary antibodies (Amersham Biosciences) and an enhanced chemiluminescence kit (Pierce).
Immune complex kinase assay
Immune complex kinase assays were performed as previously described.21, 38 In brief, cells were lysed in a lysis buffer38 and the lysates were subjected to centrifugation at 12 000 × g for 15 min at 4°C. The resulting supernatants were assayed for protein concentration with a Bradford assay kit (Bio-Rad), and equal amounts of supernatant protein were then subjected to immunoprecipitation with the indicated antibodies. The resulting immunoprecipitates were incubated for 30 min at 30°C in 15 μl of kinase reaction buffer38 containing 1 μCi of γ-32P-labelled ATP and 2 μg of substrate protein. The GST fusion proteins were used as substrates: GST-SEK1(K129R) for ASK1, MEKK1, and MLK3; GST-SAPKβ(K55R) for SEK1 and MKK7; GST-c-Jun(1–79) for JNK1; GST-ATF2 for p38; myelin basic protein (MBP) for ERK2. The reaction mixtures were subjected to SDS-PAGE and the phosphorylation of substrate proteins was analyzed with a Fuji BAS 2500 phosphorimager.
In vitro binding assay
GST fusion constructs of ASK1 deletion mutants (GST-ASK1-NT, GST-ASK1-K, and GST-ASK1-CT) were bacterially expressed and purified. Full-length Gemin5 was produced by in vitro transcription and translation in the presence of 35S-labelled methionine, using the TnT reticulocyte lysate system (Promega). 35S-labeled Gemin5 was incubated for 3 h at 4°C with each of the GST-fused ASK1 deletion mutants in a binding buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 2 mM EDTA, 1 mM DTT, 0.1% NP-40, and 5 mg/ml bovine serum albumin). The binding complexes were applied to glutathione–agarose beads, and the beads were washed three times with a solution containing 50 mM HEPES, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM DTT, and 0.1% Tween 20. Bead-bound proteins were eluted from the beads and analyzed by SDS-PAGE and autoradiography.
siRNA for Gemin5
Two different target sequences were chosen for siRNAs of human Gemin5, using the siRNA target finder program of Ambion (http://www.ambion.com): G5-siRNA1 (5′-AAACAGCTGTTACTTTCTACA-3′) and G5-siRNA2 (5′-AACCAGTTATCTGCACTCCAG-3′). For preparation of double-stranded oligonucleotides corresponding to these target sequences, the following oligonucleotides were synthesized (Bionics, Korea): 5′-GATCC ACAGCTGTTACTTTCTACATTCAAGAGATGTAGAAAGTAACAGCTGTTTTTTTGGAAA-3′ and 5′-AGCTTTTCCAAAA AAACAGCTGTTACTTTCTACATCTCTTGAATGTAGAAAGTAACAGCTGTG-3′ for G5-siRNA1 and 5′-GATCCGCCAGTTATCTGCACTCCAGTTCAAGAGACTGGAGTGCAGATAACTGGTTTTTTGGAAA-3′ and 5′-AGCTTTTCCAAAAAACCAGTTATCTGCACTCCAGTCTCTTGAACTGGAGTGCAGATAACTGGCG-3′ for G5-siRNA2. The annealed oligonucleotides were digested with BglII and HindIII, and then inserted into the BamHI and HindIII sites of the pSuper-retro vector (Oligoengine). The nucleotide sequences of the insert were confirmed by DNA sequencing. HeLa cells were stably transfected with the vectors for Gemin5 siRNAs, or with a control vector for green fluorescent protein (GFP) siRNA.39 Stable transfectants were selected in the presence of 0.2 μg/ml puromycin. Heterogeneous populations of the stably transfected cells were used to avoid clonal variation.
Site-directed mutagenesis of human Gemin5 was performed with the use of a Quickchange kit (Stratagene). A silent third-codon point mutation (TTA → TTG) within the region targeted by G5-siRNA1 was generated with the following primers: 5′-GAGGATGACAAACAGCTGTTGCTTTCTACATCAATGGAT-3′ and 5′-ATCCATTGATGTAGAAAGCAACAGCTGTTTGTCATCCTC-3′.
Apoptotic cell death
Apoptotic cell death was analyzed by DAPI staining,20 and the TUNEL method with the use of an in situ cell death detection kit (Roche Applied Science). Alternatively, apoptotic cells (Annexin V-FITC positive, propidium iodide (PI) negative) were analyzed by flow cytometry (FacsCalibur, Becton-Dickinson) and CellQuest software (BD Biosciences) after staining with Annexin V-FITC (BD Pharmingen) and PI.
Statistical analysis
Statistical significance (P-value) analysis was performed with the Student's t-test, with SPSS for windows version 12.0 (SPSS Inc., Chicago, USA).
Abbreviations
- MAPK:
-
mitogen-activated protein kinase
- ERK:
-
extracellular signal-regulated kinase
- JNK:
-
c-Jun NH2-terminal kinase
- SAPK:
-
stress-activated protein kinase
- JIP:
-
JNK-interacting protein
- JSAP1:
-
JNK/SAPK-associated protein 1
- ASK1:
-
apoptosis signal-regulating kinase 1
- GST:
-
glutathione S-transferase
- SMA:
-
spinal muscular atrophy
- SMN:
-
survival of motor neurons
- RNAi:
-
RNA interference
- HA:
-
hemagglutinin epitope
- PAGE:
-
polyacrylamide gel electrophoresis
- MBP:
-
myelin basic protein
- siRNA:
-
small interfering RNA
- GFP:
-
green fluorescent protein
- TUNEL:
-
terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling
- UV:
-
ultraviolet
References
Raman M, Cobb MH . MAP kinase modules: many roads home. Curr Biol 2003; 13: R886–R888.
Ip YT, Davis RJ . Signal transduction by the c-Jun N-terminal kinase (JNK) from inflammation to development. Curr Opin Cell Biol 1998; 10: 205–219.
Schaeffer HJ, Catling AD, Eblen ST, Collier LS, Krauss A, Weber MJ . MP1: a MEK binding partner that enhances enzymatic activation of the MAP kinase cascade. Science 1998; 281: 1668–1671.
Stewart S, Sundaram M, Zhang Y, Lee J, Han M, Guan KL . Kinase suppressor of Ras forms a multiprotein signaling complex and modulates MEK localization. Mol Cell Biol 1999; 19: 5523–5534.
Whitmarsh AJ, Cavanagh J, Tournier C, Yasuda J, Davis RJ . A mammalian scaffold complex that selectively mediates MAP kinase activation. Science 1998; 28: 1671–1674.
Ito M, Yoshioka K, Akechi M, Yamashita S, Takamatsu N, Sugiyama K et al. JSAP1, a novel jun N-terminal protein kinase (JNK)-binding protein that functions as a scaffold factor in the JNK signaling pathway. Mol Cell Biol 1999; 19: 7539–7548.
Kelkar N, Gupta S, Dickens M, Davis RJ . Interaction of a mitogen-activated protein kinase signaling module with the neuronal protein JIP3. Mol Cell Biol 2000; 20: 1030–1043.
McDonald PH, Chow CW, Miller WE, Laporte SA, Field ME, Lin FT et al. Beta-arrestin 2: a receptor-regulated MAPK scaffold for the activation of JNK3. Science 2000; 290: 1574–1577.
Nihalani D, Meyer D, Pajni S, Holzman LB . Mixed lineage kinase-dependent JNK activation is governed by interactions of scaffold protein JIP with MAPK module components. EMBO J 2001; 20: 3447–3458.
Matsuura H, Nishitoh H, Takeda K, Matsuzawa A, Amagasa T, Ito M et al. Phosphorylation-dependent scaffolding role of JSAP1/JIP3 in the ASK1–JNK signaling pathway. A new mode of regulation of the MAP kinase cascade. J Biol Chem 2002; 277: 40703–40709.
Kanamoto T, Mota M, Takeda K, Rubin LL, Miyazono K, Ichijo H et al. Role of apoptosis signal-regulating kinase in regulation of the c-Jun N-terminal kinase pathway and apoptosis in sympathetic neurons. Mol Cell Biol 2000; 20: 196–204.
Shinoda S, Skradski SL, Araki T, Schindler CK, Meller R, Lan JQ et al. Formation of a tumour necrosis factor receptor 1 molecular scaffolding complex and activation of apoptosis signal-regulating kinase 1 during seizure-induced neuronal death. Eur J Neurosci 2003; 17: 2065–2076.
Asada M, Yamada T, Ichijo H, Delia D, Miyazono K, Fukumuro K et al. Apoptosis inhibitory activity of cytoplasmic p21(Cip1/WAF1) in monocytic differentiation. EMBO J 1999; 18: 1223–1234.
Takeda K, Hatai T, Hamazaki TS, Nishitoh H, Saitoh M, Ichijo H . Apoptosis signal-regulating kinase 1 (ASK1) induces neuronal differentiation and survival of PC12 cells. J Biol Chem 2000; 275: 9805–9813.
Chang HY, Nishitoh H, Yang X, Ichijo H, Baltimore D . Activation of apoptosis signal-regulating kinase 1 (ASK1) by the adapter protein Daxx. Science 1998; 281: 1860–1863.
Nishitoh H, Saitoh M, Mochida Y, Takeda K, Nakano H, Rothe M et al. ASK1 is essential for JNK/SAPK activation by TRAF2. Mol Cell 1998; 2: 389–395.
Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y et al. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J 1998; 17: 2596–2606.
Hoeflich KP, Yeh WC, Yao Z, Mak TW, Woodgett JR . Mediation of TNF receptor-associated factor effector functions by apoptosis signal-regulating kinase-1 (ASK1). Oncogene 1999; 18: 5814–5820.
Cho SG, Lee YH, Park HS, Ryoo K, Kang KW, Park J et al. Glutathione S-transferase mu modulates the stress-activated signals by suppressing apoptosis signal-regulating kinase 1. J Biol Chem 2001; 276: 12749–12755.
Cho SG, Kim JW, Lee YH, Hwang HS, Kim MS, Ryoo K et al. Identification of a novel antiapoptotic protein that antagonizes ASK1 and CAD activities. J Cell Biol 2003; 163: 71–81.
Park HS, Lee JS, Huh SH, Seo JS, Choi EJ . Hsp72 functions as a natural inhibitory protein of c-Jun N-terminal kinase. EMBO J 2001; 20: 446–456.
Gubitz AK, Mourelatos Z, Abel L, Rappsilber J, Mann M, Dreyfuss G . Gemin5, a novel WD repeat protein component of the SMN complex that binds Sm proteins. J Biol Chem 2002; 277: 5631–5636.
Paushkin S, Gubitz AK, Massenet S, Dreyfuss G . The SMN complex, an assemblyosome of ribonucleoproteins. Curr Opin Cell Biol 2002; 14: 305–312.
Smith TF, Gaitatzes C, Saxena K, Neer EJ . The WD repeat: a common architecture for diverse functions. Trends Biochem Sci 1999; 24: 181–185.
Li D, Roberts R . WD-repeat proteins: structure characteristics, biological function, and their involvement in human diseases. Cell Mol Life Sci 2001; 58: 2085–2097.
Beere HM, Green DR . Stress management – heat shock protein-70 and the regulation of apoptosis. Trends Cell Biol 2001; 11: 6–10.
Teramoto H, Coso OA, Miyata H, Igishi T, Miki T, Gutkind JS . Signaling from the small GTP-binding proteins Rac1 and Cdc42 to the c-Jun N-terminal kinase/stress-activated protein kinase pathway. A role for mixed lineage kinase 3/protein-tyrosine kinase 1, a novel member of the mixed lineage kinase family. J Biol Chem 1996; 271: 27225–27228.
Gotoh Y, Cooper JA . Reactive oxygen species- and dimerization-induced activation of apoptosis signal-regulating kinase 1 in tumor necrosis factor-alpha signal transduction. J Biol Chem 1998; 273: 17477–17482.
Liu H, Nishitoh H, Ichijo H, Kyriakis JM . Activation of apoptosis signal-regulating kinase 1 (ASK1) by tumor necrosis factor receptor-associated factor 2 requires prior dissociation of the ASK1 inhibitor thioredoxin. Mol Cell Biol 2000; 20: 2198–2208.
Tournier C, Whitmarsh AJ, Cavanagh J, Barrett T, Davis RJ . Mitogen-activated protein kinase kinase 7 is an activator of the c-Jun NH2-terminal kinase. Proc Natl Acad Sci USA 1997; 94: 7337–7342.
Tobiume K, Matsuzawa A, Takahashi T, Nishitoh H, Morita K, Takeda K et al. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep 2001; 2: 222–228.
Pellizzoni L, Kataoka N, Charroux B, Dreyfuss G . A novel function for SMN, the spinal muscular atrophy disease gene product, in pre-mRNA splicing. Cell 1998; 95: 615–624.
Nicole S, Diaz CC, Frugier T, Melki J . Spinal muscular atrophy: recent advances and future prospects. Muscle Nerve 2002; 26: 4–13.
Lefebvre S, Burglen L, Reboullet S, Clermont O, Burlet P, Viollet L et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell 1995; 80: 155–165.
Gubitz AK, Feng W, Dreyfuss G . The SMN complex. Exp Cell Res 2004; 296: 51–56.
Liu Q, Dreyfuss G . A novel nuclear structure containing the survival of motor neurons protein. EMBO J 1996; 15: 3555–3565.
Park HS, Yu JW, Cho JH, Kim MS, Huh SH, Ryoo K et al. Inhibition of apoptosis signal-regulating kinase 1 (ASK1) by nitric oxide through a thiol-redox mechanism. J Biol Chem 2004; 279: 7584–7590.
Ryoo K, Huh SH, Lee YH, Yoon KW, Cho SG, Choi EJ . Negative regulation of MEKK1-induced signaling by glutathione S-transferase mu. J Biol Chem 2004; 279: 43589–43594.
Hamar P, Song E, Kokeny G, Chen A, Ouyang N, Lieberman J . Small interfering RNA targeting Fas protects mice against renal ischemia-reperfusion injury. Proc Natl Acad Sci USA 2004; 101: 14883–14888.
Acknowledgements
We thank Drs Ichijo H, Davis RJ, Woodgett J, Zon LI, Yoshioka K, Cobb MH, Ulevitch RJ, and Gutkind JS for providing ASK1, JNK1, SAPK, SEK1, Flag-SEK1, ERK2, p38, and MLK3 cDNA clones, respectively. EK Kim is a recipient of BK21 postdoctoral fellowship. This work was supported by the Molecular and Cellular BioDiscovery Research Program grant (M10601000136-06N0100-13610) from the Korean Ministry of Science and Technology, and by the Korea Research Foundation Grant (KRF-2006-341-C00023) funded by the Korean Government (MOEHRD) (E-JC).
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by H Ichijo
Rights and permissions
About this article
Cite this article
Kim, E., Noh, K., Yoon, JH. et al. Positive regulation of ASK1-mediated c-Jun NH2-terminal kinase signaling pathway by the WD-repeat protein Gemin5. Cell Death Differ 14, 1518–1528 (2007). https://doi.org/10.1038/sj.cdd.4402157
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cdd.4402157
Keywords
This article is cited by
-
Arginine methylation-dependent regulation of ASK1 signaling by PRMT1
Cell Death & Differentiation (2012)
-
ASK1
AfCS-Nature Molecule Pages (2010)