Abstract
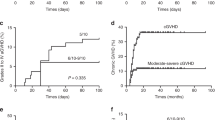
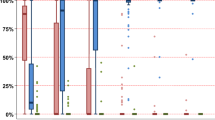
Early results of unrelated cord blood transplantation (UCBT) for severe aplastic anemia (SAA) were poor with a high rate of engraftment failure. This was attributed to the combination of lower graft cell dose and intact host immune system. We performed UCBT in nine children (median age 9 years) with refractory SAA using increasingly immunosuppressive preparative regimens. The time from diagnosis to UCBT was 3.4–20 months (median age 7.2 years), with all children having failed at least one course of immunosuppression. Donor/recipient HLA matching was six of six (n=1), five of six (n=2) and four of six (n=6). The median nucleated cell dose infused was 5.7 × 107 cells/kg (range 3.5–20 × 107 cells/kg). Six patients were engrafted after the first UCBT. Two of the three patients without hematopoietic reconstitution were engrafted after a second UCBT. All children receiving ⩾120 mg/kg of CY in the preparative regimen were engrafted. The median time to myeloid engraftment was 25 (17–59 days) days. Acute GVHD developed in two, and chronic GVHD in five patients. Five patients developed EBV viremia post transplant (lymphoproliferative disorder in three patients). At a median follow-up of 34 months, seven patients are alive and transfusion-independent. UCBT is a feasible treatment strategy for children with refractory SAA lacking a well-matched adult donor.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Kojima S, Hibi S, Kosaka Y, Yamamoto M, Tsuchida M, Mugishima H et al. Immunosuppressive therapy using antithymocyte globulin, cyclosporine, and danazol with or without human granulocyte colony-stimulating factor in children with acquired aplastic anemia. Blood 2000; 96: 2049–2054.
Bacigalupo A, Bruno B, Saracco P, Di Bona E, Locasciulli A, Locatelli F et al. Antithymocyte globulin, cyclosporine, prednisolone, and granulocyte colony-stimulating factor for severe aplastic anemia: an update of the GITMO/EBMT study of 100 patients. European Group for Blood and Marrow Transplantation (EBMT) Working Party on Severe Aplastic Anemia and the Gruppo Italino Transplanti di Midolio Osseo. Blood 2000; 95: 1931–1934.
Fuhrer M, Rampf U, Baumann I, Faldum A, Niemeyer C, Janka-Schaub G et al. Immunosuppressive therapy for aplastic anemia in children: a more severe disease predicts better survival. Blood 2005; 106: 2102–2104.
Kojima S, Horibe K, Inaba J, Yashimi A, Takahashi Y, Kudo K et al. Long-term outcome of acquired aplastic anemia in children: comparison between immunosuppressive therapy and bone marrow transplantation. Br J Haematol 2000; 111: 321–328.
Scheinberg P, Nunez O, Young NS . Retreatment with rabbit anti-thymocyte globulin and cyclosporine for patients with relapsed or refractory severe aplastic anemia. Br J Haematol 2006; 113: 622–627.
Kasaka Y, Yagasaki H, Sano K, Kobayashi R, Ayukawa H, Kaneko T et al. Prospective multicenter trial comparing repeated immunosuppressive therapy with stem-cell transplantation from an alternative donor as second-line treatment of children with severe and very severe aplastic anemia. Blood 2008; 111: 1054–1059.
Locasciulli A, Oneto R, Bacigalupo A, Socie G, Korthof E, Bekassy A et al. Outcome of patients with acquired aplastic anemia given first line bone marrow transplantation or immunosuppressive treatment in the last decade: a report from the European Group for Blood and Marrow Transplantation. Haematologica 2007; 92: 11–18.
Deeg HJ, O’Donnell M, Tolar J, Agarwal R, Harris R, Feig SA et al. Optimization of conditioning for marrow transplantation from unrelated donors for patients with aplastic anemia after failure of immunosuppressive therapy. Blood 2006; 108: 1485–1491.
Kojima S, Matasuygama T, Kato S, Kigasawa H, Kobayashi R, Kikuta A et al. Outcome of 154 patients with severe aplastic anemia who received transplants from unrelated donors: the Japan Marrow Donor Program. Blood 2002; 100: 799–803.
Maury S, Balere-Appert ML, Chir Z, Boiron JM, Galambrun C, Yakouben K et al. Unrelated stem cell transplantation for severe acquired aplastic anemia: improved outcome in the era of high-resolution HLA matching between donor and recipient. Haematologica 2007; 92: 589–596.
Viollier R, Socie G, Tichelli A, Bacigalupo A, Korthof ET, Marsh J et al. Recent improvement in outcomes of unrelated donor transplantation for aplastic anemia. Bone Marrow Transplant 2008; 41: 45–50.
Marsh JCW, Ball SE, Darbyshire P, Gordon-Smith EC, Keidan AJ, Martin A et al. Guidelines for the diagnosis and management of acquired aplastic anemia. Br J Haematol 2003; 123: 782–801.
Hurley CK, Baxter Lowe LA, Logan B, Karanes C, Anasetti C, Weisdorf W et al. National marrow donor program HLA-matching guidelines for unrelated donor transplants. Biol Blood Marrow Transplant 2003; 9: 610–615.
Passweg JR, Perez WS, Eapen M, Camitta BM, Gluckman E, Hinterberger W et al. Bone marrow transplants from mismatched related and unrelated donors for severe aplastic anemia. Bone Marrow Transplant 2006; 37: 641–649.
Eapen M, Rubinstein P, Zhang MJ, Stevens C, Kurtzberg J, Scaradavou A et al. Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow transplants in children with acute leukemia: a comparison study. Lancet 2007; 369: 1947–1954.
Rubinstein P, Carrier C, Scaradavou A, Kurtzberg J, Adamson J, Migliaccio AR et al. Outcome among 562 recipients of placental-blood transplants from unrelated donors. N Engl J Med 1998; 339: 1565–1577.
Kojima S, Yoshimi A, Taniguchi S, Hara J, Matsui T, Kato S et al. Unrelated cord blood transplantation for acquired severe aplastic anemia: the report from the Japan Cord Blood Bank Network. Blood 2007; 118: 601a (abstract 2020).
Ohga S, Ichino K, Goto K, Hattori S, Nomura A, Takada H et al. Unrelated donor cord blood transplantation for childhood severe aplastic anemia after modified conditioning. Pediatr Transplant 2006; 10: 497–500.
Mao P, Zhu Z, Wang H, Wang S, Mo W, Ying Y et al. Sustained and stable hematopoietic donor–recipient mixed chimerism after unrelated cord blood transplantation for adult patients with severe aplastic anemia. Eur J Haematol 2005; 75: 430–435.
Tajika K, Mizuki T, Nakayama K, Yamaguchi H, Dan K . Umbilical blood cell transplantation conditioned with a reduced intensity-regimen is a practical salvage therapy for severe aplastic anemia refractory to immunosuppressive therapy with antithymocyte globulin/ciclosporin. J Nippon Med Sch 2007; 74: 424–429.
Lim SH, Zhang Y, Wang Z, Varadarajan R, Smith P, Burris C et al. Umbilical cord blood transplant in hepatitis C-associated severe aplastic anemia. Bone Marrow Transplant 2004; 33: 565–567.
International Agranulocytosis and Aplastic anemia Study Group. Incidence of aplastic anemia: the relevance of diagnostic criteria. Blood 1987; 70: 1718–1721.
Maiers M, Gragert L, Klintz W . Predicting high-resolution match rates for hematopoietic stem cell transplant patients using four-locus KLA haplotypes. Human Immunol 2006; 67: 30.
Benesch M, Urban C, Sykora K, Schwinger W, Zintl F, Lackner H et al. Transplantation of highly purified CD34+ progenitor cells from alternative donors in children with refractory severe aplastic anemia. Br J Haeamtol 2004; 125: 58–63.
Woodard P, Cunningham J, Benaim E, Chen X, Hale G, Horwitz E et al. Effective donor lymhohematopoietic reconstitution after haploidentical CD34+-selected hematopoietic stem cell transplantation in children with refractory severe aplastic anemia. Bone Marrow Transplant 2004; 33: 411–418.
Bunin N, Aplenc R, Iannone R, Leahey A, Grupp S, Monos D et al. Unrelated donor bone marrow transplants for children with severe aplastic anemia: minimal GVHD and durable engraftment with partial T cell depletion. Bone marrow Transplant 2005; 35: 369–373.
De Latour RP, Rocha V, Robin M, Rodrigues CA, Rea D, Laghero J et al. Double cord blood transplantation in bone marrow failure syndromes. Blood 2007; 118: 601a (abstract 2018).
Yagasaki H, Takahashi Y, Kudo K, Ohashi H, Hama A, Yamamoto T et al. Feasibility and results of bone marrow transplantation from an HLA-mismatched unrelated donor for children and young adults with acquired aplastic anemia. Int J Hematol 2007; 85: 437–442.
Inagaski J, Nagatoshi Y, Kawano Y, Saito Y, Takahashi D, Nagayama J et al. Bon marrow transplantation in children with severe aplastic anemia using a conditioning regimen containing 3 Gy of total body irradiation, cyclophosphamide with or without antithymocyte globulin. Pediatr Transplantation 2007; 11: 180–186.
Srinivasan R, Takahashi Y, McCoy JP, Espinoza-Delago I, Dorrance C, Igarashi T et al. Overcoming graft rejection in heavily transfused and allo-immunized patients with bone marrow failure syndromes using fludarabine-based haematopoietic cell transplantation. Br J Haematol 2006; 133: 305–311.
Bacigalupo A, Locaatelli F, Lanino E, Marsh J, Socie G, Maury S et al. Fludarabine, cyclophosphamide and anti-thymocyte globulin for alternative donor transplantation in acquired aplastic anemia: a report from the EBMT-SAA working party. Bone Marrow Transplant 2005; 36: 947–950.
Antin JH, Childs R, Filipovich AH, Giralt S, MacKinnon S, Spitzer T et al. Establishment of complete and mixed donor chimerism after allogeneic lymphohematopoietic transplantation: recommendations from a workshop at the 2001 Tandem Meetings. Biol Blood Marrow Transplant 2001; 7: 473–485.
Marsh JCW . Treatment of acquired aplastic anemia. Haematologica 2007; 91: 1–5.
Hurley CK, Wagner JE, Setterholm MI, Confer DL . Advances in HLA: practical implications for selecting adult donors and cord blood units. Biol Blood Marrow Transplant 2006; 12: 28–33.
Brunstein CG, Weisdorf DJ, DeFor T, Barker JN, Tolar J, van Burik J-A H et al. Marked increased risk of Epstein-Barr virus-related complications with the addition of antithymocyte globulin to a nonmyeloablative conditioning prior to unrelated umbilical cord blood transplantation. Blood 2006; 108: 2874–2880.
Scheinberg P, Fischeer SH, Li L, Nunez O, Wu CO, Sloand EM et al. Distinct EBV and CMV reactivation patterns following antibody-based immunosuppressive regimens in patients with severe aplastic anemia. Blood 2007; 109: 3219–3224.
Van Esser JW, Niesters HGM, van der Holt B, Meijer E, Osterhaus ADME, Gratama JW et al. Prevention of Epstein-Barr virus-lymphoproliferative disease by molecular monitoring and preemptive rituximab in high-risk patients after allogeneic stem cell transplantation. Blood 2002; 99: 4364–4369.
Kennedy-Nasser AA, Leung KS, Mahajan A, Weiss HL, Arce JA, Gottschalk S et al. Comparable outcomes of matched-related and alternative donor stem cell transplantation for pediatric severe aplastic anemia. Biol Blood Marrow Transplant 2006; 12: 1277–1284.
Gupta V, Ball SE, Sage D, Ortin M, Freires M, Gordon-Smith EC et al. Marrow transplants from unrelated donors for aplastic anemia using alemtuzumab, fludarabine and cyclophosphamide based conditioning. Bone Marrow Transplant 2005; 35: 467–471.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chan, K., McDonald, L., Lim, D. et al. Unrelated cord blood transplantation in children with idiopathic severe aplastic anemia. Bone Marrow Transplant 42, 589–595 (2008). https://doi.org/10.1038/bmt.2008.227
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2008.227
Keywords
This article is cited by
-
Unrelated cord blood transplantation for severe aplastic anemia using intensified immunoablative conditioning regimen leading to high engraftment and survival
Bone Marrow Transplantation (2020)
-
Immunosuppressive therapy versus alternative donor hematopoietic stem cell transplantation for children with severe aplastic anemia who lack an HLA-matched familial donor
Bone Marrow Transplantation (2017)
-
Alternative donor transplant of benign primary hematologic disorders
Bone Marrow Transplantation (2015)
-
Unrelated cord blood transplantation for newly diagnosed patients with severe acquired aplastic anemia using a reduced-intensity conditioning: high graft rejection, but good survival
Bone Marrow Transplantation (2012)
-
Is it time for a change? The case for early application of unrelated allo-SCT for severe aplastic anemia
Bone Marrow Transplantation (2010)