Abstract
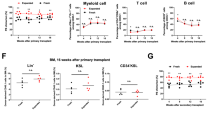
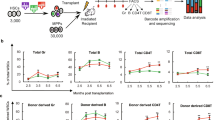
In this study, we use competitive repopulation to compare the quality and frequency of stem cells isolated from mobilized blood with stem cells isolated from bone marrow (BM) in a mouse model. Lin−Sca-1+c-Kit+ (LSK) cells were harvested from control BM and peripheral blood of mice following granulocyte colony-stimulating factor (G-CSF) administration. LSK cells were used because of their resemblance to human CD34+ cells. We confirmed that transplantation of phenotypically defined mobilized peripheral blood (MPB) stem cells results in rapid recovery of blood counts. However, in vitro results indicated that LSK cells purified from MPB had lower cobblestone area-forming cell day 35 activity compared to BM. Additionally, evaluation of chimerism after co-transplantation of LSK cells purified from blood and BM revealed that MPB stem cells contained 25-fold less repopulation potential compared to BM stem cells. Competitive repopulating unit frequency analysis showed that freshly isolated MPB LSK cells have 8.8-fold fewer cells with long-term repopulating ability compared to BM LSK cells. Secondary transplantation showed no further decline in contribution of hematopoiesis relative to BM. We conclude that the reduced frequency of stem cells within the LSK population of MPB, rather than poorer quality, causes the reduced repopulation potential.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Mathe G, Thomas ED, Ferrebree JW . The restoration of marrow function after lethal irradiation in man: a review. Transplant Bull 1959; 6: 407–409.
Goldman J . Peripheral blood stem cells for allografting. Blood 1995; 85: 1413–1415.
Korbling M, Champlin R . Peripheral blood progenitor cell transplantation: a replacement for marrow auto- or allografts. Stem Cells 1996; 14: 185–195.
To LB, Haylock DN, Simmons PJ, Juttner CA . The biology and clinical uses of blood stem cells. Blood 1997; 89: 2233–2258.
Varas F, Bernad A, Bueren JA . Granulocyte colony-stimulating factor mobilizes into peripheral blood the complete clonal repertoire of hematopoietic precursors residing in the bone marrow of mice. Blood 1996; 88: 2495–2501.
Molineux G, Pojda Z, Dexter TM . A comparison of hematopoiesis in normal and splenectomized mice treated with granulocyte colony-stimulating factor. Blood 1990; 75: 563–569.
Yan XQ, Hartley C, McElroy P, Chang A, McCrea C, McNiece I . Peripheral blood progenitor cells mobilized by recombinant human granulocyte colony-stimulating factor plus recombinant rat stem cell factor contain long-term engrafting cells capable of cellular proliferation for more than two years as shown by serial transplantation in mice. Blood 1995; 85: 2303–2307.
Demuynck H, Pettengell R, de Campos E, Dexter TM, Testa NG . The capacity of peripheral blood stem cells mobilised with chemotherapy plus G-CSF to repopulate irradiated marrow stroma in vitro is similar to that of bone marrow. Eur J Cancer 1992; 28: 381–386.
Pettengell R, Luft T, Henschler R, Hows JM, Dexter TM, Ryder D et al. Direct comparison by limiting dilution analysis of long-term culture-initiating cells in human bone marrow, umbilical cord blood, and blood stem cells. Blood 1994; 84: 3653–3659.
Molineux G, McCrea C, Yan XQ, Kerzic P, McNiece I . Flt-3 ligand synergizes with granulocyte colony-stimulating factor to increase neutrophil numbers and to mobilize peripheral blood stem cells with long-term repopulating potential. Blood 1997; 89: 3998–4004.
Wagers AJ, Allsopp RC, Weissman IL . Changes in integrin expression are associated with altered homing properties of Lin(-/lo)Thy1.1(lo)Sca-1(+)c-kit(+) hematopoietic stem cells following mobilization by cyclophosphamide/granulocyte colony-stimulating factor. Exp Hematol 2002; 30: 176–185.
Szilvassy SJ, Meyerrose TE, Ragland PL, Grimes B . Differential homing and engraftment properties of hematopoietic progenitor cells from murine bone marrow, mobilized peripheral blood, and fetal liver. Blood 2001; 98: 2108–2115.
Uchida N, He D, Friera AM, Reitsma M, Sasaki D, Chen B et al. The unexpected G0/G1 cell cycle status of mobilized hematopoietic stem cells from peripheral blood. Blood 1997; 89: 465–472.
de Haan G, Ausema A, Wilkens M, Molineux G, Dontje B . Efficient mobilization of haematopoietic progenitors after a single injection of pegylated recombinant human granulocyte colony-stimulating factor in mouse strains with distinct marrow-cell pool sizes. Br J Haematol 2000; 110: 638–646.
de Haan G, Szilvassy SJ, Meyerrose TE, Dontje B, Grimes B, Van Zant G . Distinct functional properties of highly purified hematopoietic stem cells from mouse strains differing in stem cell numbers. Blood 2000; 96: 1374–1379.
Szilvassy SJ, Humphries RK, Lansdorp PM, Eaves AC, Eaves CJ . Quantitative assay for totipotent reconstituting hematopoietic stem cells by a competitive repopulation strategy. Proc Natl Acad Sci USA 1990; 87: 8736–8740.
Ploemacher RE, van der Sluijs JP, Voerman JS, Brons NH . An in vitro limiting-dilution assay of long-term repopulating hematopoietic stem cells in the mouse. Blood 1989; 74: 2755–2763.
Yeoh JS, van Os R, Weersing E, Ausema A, Dontje B, Vellenga E et al. Fibroblast growth factor-1 and 2 preserve long-term repopulating ability of hematopoietic stem cells in serum-free cultures. Stem Cells 2006; 24: 1564–1572.
de Haan G, Van Zant G . Intrinsic and extrinsic control of hemopoietic stem cell numbers: mapping of a stem cell gene. J Exp Med 1997; 186: 529–536.
Ploemacher RE, van der Sluijs JP, van Beurden CA, Baert MR, Chan PL . Use of limiting-dilution type long-term marrow cultures in frequency analysis of marrow-repopulating and spleen colony-forming hematopoietic stem cells in the mouse. Blood 1991; 78: 2527–2533.
Levesque JP, Hendy J, Winkler IG, Takamatsu Y, Simmons PJ . Granulocyte colony-stimulating factor induces the release in the bone marrow of proteases that cleave c-KIT receptor (CD117) from the surface of hematopoietic progenitor cells. Exp Hematol 2003; 31: 109–117.
Ikuta K, Weissman IL . Evidence that hematopoietic stem cells express mouse c-kit but do not depend on steel factor for their generation. Proc Natl Acad Sci USA 1992; 89: 1502–1506.
Okada S, Nakauchi H, Nagayoshi K, Nishikawa S, Nishikawa S, Miura Y et al. Enrichment and characterization of murine hematopoietic stem cells that express c-kit molecule. Blood 1991; 78: 1706–1712.
Roberts AW, Foote S, Alexander WS, Scott C, Robb L, Metcalf D . Genetic influences determining progenitor cell mobilization and leukocytosis induced by granulocyte colony-stimulating factor. Blood 1997; 89: 2736–2744.
Tajima F, Sato T, Laver JH, Ogawa M . CD34 expression by murine hematopoietic stem cells mobilized by granulocyte colony-stimulating factor. Blood 2000; 96: 1989–1993.
Osawa M, Hanada K, Hamada H, Nakauchi H . Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science 1996; 273: 242–245.
Morrison SJ, Hemmati HD, Wandycz AM, Weissman IL . The purification and characterization of fetal liver hematopoietic stem cells. Proc Natl Acad Sci USA 1995; 92: 10302–10306.
Morrison SJ, Wandycz AM, Akashi K, Globerson A, Weissman IL . The aging of hematopoietic stem cells. Nat Med 1996; 2: 1011–1016.
Morrison SJ, Wright DE, Weissman IL . Cyclophosphamide/granulocyte colony-stimulating factor induces hematopoietic stem cells to proliferate prior to mobilization. Proc Natl Acad Sci USA 1997; 94: 1908–1913.
Yilmaz OH, Kiel MJ, Morrison SJ . SLAM family markers are conserved among hematopoietic stem cells from old and reconstituted mice and markedly increase their purity. Blood 2006; 107: 924–930.
To LB, Roberts MM, Haylock DN, Dyson PG, Branford AL, Thorp D et al. Comparison of haematological recovery times and supportive care requirements of autologous recovery phase peripheral blood stem cell transplants, autologous bone marrow transplants and allogeneic bone marrow transplants. Bone Marrow Transplant 1992; 9: 277–284.
Wierenga PK, Setroikromo R, Kamps G, Kampinga HH, Vellenga E . Peripheral blood stem cells differ from bone marrow stem cells in cell cycle status, repopulating potential, and sensitivity toward hyperthermic purging in mice mobilized with cyclophosphamide and granulocyte colony-stimulating factor. J Hematother Stem Cell Res 2002; 11: 523–532.
Korbling M, Anderlini P . Peripheral blood stem cell versus bone marrow allotransplantation: does the source of hematopoietic stem cells matter? Blood 2001; 98: 2900–2908.
Bender JG, To LB, Williams S, Schwartzberg LS . Defining a therapeutic dose of peripheral blood stem cells. J Hematother 1992; 1: 329–341.
Kaufman CL, Colson YL, Wren SM, Watkins S, Simmons RL, Ildstad ST . Phenotypic characterization of a novel bone marrow-derived cell that facilitates engraftment of allogeneic bone marrow stem cells. Blood 1994; 84: 2436–2446.
Acknowledgements
We thank Geert Mesander and Henk Moes for their assistance with cell sorting and the staff of our animal facility for taking care of the mice. No conflicts of interest exists between authors. This work was supported by Grants from the: European Union (EU-LSHC-CT-2004–503436) and the Ubbo Emmius Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yeoh, J., Ausema, A., Wierenga, P. et al. Mobilized peripheral blood stem cells provide rapid reconstitution but impaired long-term engraftment in a mouse model. Bone Marrow Transplant 39, 401–409 (2007). https://doi.org/10.1038/sj.bmt.1705601
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1705601
Keywords
This article is cited by
-
New agents in HSC mobilization
International Journal of Hematology (2017)
-
Therapeutic targeting and rapid mobilization of endosteal HSC using a small molecule integrin antagonist
Nature Communications (2016)
-
Bone marrow-derived cells can acquire renal stem cells properties and ameliorate ischemia-reperfusion induced acute renal injury
BMC Nephrology (2012)