Abstract
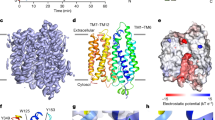
ClC chloride channels and transporters are important for chloride homeostasis in species from bacteria to human. Mutations in ClC proteins cause genetically inherited diseases, some of which are likely to involve folding defects. The ClC proteins present a challenging and unusual biological folding problem because they are large membrane proteins possessing a complex architecture, with many reentrant helices that go only partway through membrane and loop back out. Here we were able to examine the unfolding of the Escherichia coli ClC transporter, ClC-ec1, using single-molecule forced unfolding methods. We found that the protein could be separated into two stable halves that unfolded independently. The independence of the two domains is consistent with an evolutionary model in which the two halves arose from independently folding subunits that later fused together. Maintaining smaller folding domains of lesser complexity within large membrane proteins may be an advantageous strategy to avoid misfolding traps.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Miller, C. Open-state substructure of single chloride channels from Torpedo electroplax. Phil. Trans. R. Soc. Lond. B 299, 401–411 (1982).
Accardi, A. & Miller, C. Secondary active transport mediated by a prokaryotic homologue of ClC Cl– channels. Nature 427, 803–807 (2004).
Pusch, M. et al. Mechanisms of block of muscle type CLC chloride channels (Review). Mol. Membr. Biol. 19, 285–292 (2002).
Gouaux, E. & Mackinnon, R. Principles of selective ion transport in channels and pumps. Science 310, 1461–1465 (2005).
Chen, T. Y. Structure and function of CLC channels. Annu. Rev. Physiol. 67, 809–839 (2005).
Miller, C. ClC chloride channels viewed through a transporter lens. Nature 440, 484–489 (2006).
Dutzler, R. A structural perspective on ClC channel and transporter function. FEBS Lett. 581, 2839–2844 (2007).
Jentsch, T. J. CLC chloride channels and transporters: from genes to protein structure, pathology and physiology. Crit. Rev. Biochem. Mol. Biol. 43, 3–36 (2008).
Stölting, G., Fischer, M. & Fahlke, C. CLC channel function and dysfunction in health and disease. Front. Physiol. 5, 378 (2014).
Accardi, A. Structure and gating of CLC channels and exchangers. J. Physiol. (Lond.) 593, 4129–4138 (2015).
Ludwig, M. et al. Functional evaluation of Dent’s disease-causing mutations: implications for ClC-5 channel trafficking and internalization. Hum. Genet. 117, 228–237 (2005).
Peng, Y. J. et al. Regulation of CLC-1 chloride channel biosynthesis by FKBP8 and Hsp90β. Sci. Rep. 6, 32444–32458 (2016).
Robertson, J. L., Kolmakova-Partensky, L. & Miller, C. Design, function and structure of a monomeric ClC transporter. Nature 468, 844–847 (2010).
Chadda, R. et al. The dimerization equilibrium of a ClC Cl–/H+ antiporter in lipid bilayers. Elife 5, e17438 (2016).
Mindell, J. A., Maduke, M., Miller, C. & Grigorieff, N. Projection structure of a ClC-type chloride channel at 6.5 Å resolution. Nature 409, 219–223 (2001).
Dutzler, R., Campbell, E. B., Cadene, M., Chait, B. T. & MacKinnon, R. X-ray structure of a ClC chloride channel at 3.0 Å reveals the molecular basis of anion selectivity. Nature 415, 287–294 (2002).
Dutzler, R., Campbell, E. B. & MacKinnon, R. Gating the selectivity filter in ClC chloride channels. Science 300, 108–112 (2003).
Park, E., Campbell, E. B. & MacKinnon, R. Structure of a CLC chloride ion channel by cryo-electron microscopy. Nature 541, 500–505 (2017).
von Heijne, G. Membrane-protein topology. Nat. Rev. Mol. Cell Biol. 7, 909–918 (2006).
Bowie, J. U. Flip-flopping membrane proteins. Nat. Struct. Mol. Biol. 13, 94–96 (2006).
Forrest, L. R. Structural symmetry in membrane proteins. Annu. Rev. Biophys. 44, 311–337 (2015).
Bowie, J. U. Membrane protein twists and turns. Science 339, 398–399 (2013).
Oesterhelt, F. et al. Unfolding pathways of individual bacteriorhodopsins. Science 288, 143–146 (2000).
Kedrov, A., Janovjak, H., Sapra, K. T. & Müller, D. J. Deciphering molecular interactions of native membrane proteins by single-molecule force spectroscopy. Annu. Rev. Biophys. Biomol. Struct. 36, 233–260 (2007).
Zocher, M. et al. Single-molecule force spectroscopy from nanodiscs: an assay to quantify folding, stability, and interactions of native membrane proteins. ACS Nano 6, 961–971 (2012).
Serdiuk, T. et al. YidC assists the stepwise and stochastic folding of membrane proteins. Nat. Chem. Biol. 12, 911–917 (2016).
Popot, J. L. & Engelman, D. M. Helical membrane protein folding, stability, and evolution. Annu. Rev. Biochem. 69, 881–922 (2000).
Engelman, D. M. et al. Membrane protein folding: beyond the two stage model. FEBS Lett. 555, 122–125 (2003).
Bowie, J. U. Solving the membrane protein folding problem. Nature 438, 581–589 (2005).
Kim, K. & Saleh, O. A. A high-resolution magnetic tweezer for single-molecule measurements. Nucleic Acids Res. 37, e136 (2009).
Ding, F. et al. Single-molecule mechanical identification and sequencing. Nat. Methods 9, 367–372 (2012).
De Vlaminck, I. & Dekker, C. Recent advances in magnetic tweezers. Annu. Rev. Biophys. 41, 453–472 (2012).
Min, D. et al. Mechanical unzipping and rezipping of a single SNARE complex reveals hysteresis as a force-generating mechanism. Nat. Commun. 4, 1705 (2013).
Kemmerich, F. E. et al. Simultaneous single-molecule force and fluorescence sampling of DNA nanostructure conformations using magnetic tweezers. Nano Lett. 16, 381–386 (2016).
Berghuis, B. A., Köber, M., van Laar, T. & Dekker, N. H. High-throughput, high-force probing of DNA-protein interactions with magnetic tweezers. Methods 105, 90–98 (2016).
Min, D., Jefferson, R. E., Bowie, J. U. & Yoon, T. Y. Mapping the energy landscape for second-stage folding of a single membrane protein. Nat. Chem. Biol. 11, 981–987 (2015).
Min, D., Arbing, M. A., Jefferson, R. E. & Bowie, J. U. A simple DNA handle attachment method for single molecule mechanical manipulation experiments. Protein Sci. 25, 1535–1544 (2016).
Jefferson, R. E., Min, D., Corin, K., Wang, J. Y. & Bowie, J. U. Applications of single-molecule methods to membrane protein folding studies. J. Mol. Biol. 430, 424–437 (2018).
Zakeri, B. et al. Peptide tag forming a rapid covalent bond to a protein, through engineering a bacterial adhesin. Proc. Natl Acad. Sci. USA 109, E690–E697 (2012).
Faham, S. & Bowie, J. U. Bicelle crystallization: a new method for crystallizing membrane proteins yields a monomeric bacteriorhodopsin structure. J. Mol. Biol. 316, 1–6 (2002).
Strick, T. R., Allemand, J. F., Bensimon, D., Bensimon, A. & Croquette, V. The elasticity of a single supercoiled DNA molecule. Science 271, 1835–1837 (1996).
Gosse, C. & Croquette, V. Magnetic tweezers: micromanipulation and force measurement at the molecular level. Biophys. J. 82, 3314–3329 (2002).
Ribeck, N. & Saleh, O. A. Multiplexed single-molecule measurements with magnetic tweezers. Rev. Sci. Instrum. 79, 094301–094306 (2008).
Hanggi, P., Talkner, P. & Borkovec, M. Reaction-rate theory — 50 years after Kramers. Rev. Mod. Phys. 62, 251–341 (1990).
Huang, J. & MacKerell, A. D. Jr. CHARMM36 all-atom additive protein force field: validation based on comparison to NMR data. J. Comput. Chem. 34, 2135–2145 (2013).
Zhu, L., Kaback, H. R. & Dalbey, R. E. YidC protein, a molecular chaperone for LacY protein folding via the SecYEG protein machinery. J. Biol. Chem. 288, 28180–28194 (2013).
Kumazaki, K. et al. Crystal structure of Escherichia coli YidC, a membrane protein chaperone and insertase. Sci. Rep. 4, 7299 (2014).
Dill, K. A. Theory for the folding and stability of globular proteins. Biochemistry 24, 1501–1509 (1985).
Shen, M. Y., Davis, F. P. & Sali, A. The optimal size of a globular protein domain: a simple sphere-packing model. Chem. Phys. Lett. 405, 224–228 (2005).
Paslawski, W. et al. Cooperative folding of a polytopic α-helical membrane protein involves a compact N-terminal nucleus and nonnative loops. Proc. Natl Acad. Sci. USA 112, 7978–7983 (2015).
Jefferson, R. E., Blois, T. M. & Bowie, J. U. Membrane proteins can have high kinetic stability. J. Am. Chem. Soc. 135, 15183–15190 (2013).
Blommel, P. G. & Fox, B. G. A combined approach to improving large-scale production of tobacco etch virus protease. Protein Expr. Purif. 55, 53–68 (2007).
Sreerama, N. & Woody, R. W. On the analysis of membrane protein circular dichroism spectra. Protein Sci. 13, 100–112 (2004).
Wu, E. L. et al. CHARMM-GUI Membrane Builder toward realistic biological membrane simulations. J. Comput. Chem. 35, 1997–2004 (2014).
Steinbach, P. J. & Brooks, B. R. New spherical-cutoff methods for long-range forces in macromolecular simulation. J. Comput. Chem. 15, 667–683 (1994).
Essmann, U. et al. A smooth particle mesh Ewald method. J. Chem. Phys. 103, 8577–8593 (1995).
Feller, S. E., Zhang, Y. H., Pastor, R. W. & Brooks, B. R. Constant-pressure molecular-dynamics simulation — the Langevin piston method. J. Chem. Phys. 103, 4613–4621 (1995).
Shaw, D. E. et al. Anton 2: Raising the bar for performance and programmability in a special-purpose molecular dynamics supercomputer. Sc14: Intl. Conf. High Performance Computing, Networking, Storage and Analysis 41–53 (2014).
Lippert, R. A. et al. Accurate and efficient integration for molecular dynamics simulations at constant temperature and pressure. J. Chem. Phys. 139, 164106–164116 (2013).
Hess, H. H. & Derr, J. E. Assay of inorganic and organic phosphorus in the 0.1–5 nanomole range. Anal. Biochem. 63, 607–613 (1975).
Acknowledgements
This work was supported by the National Institutes of Health (R01GM063919 to J.U.B. and U54GM087519 to W.I.), the National Science Foundation (MCB-1727508 to W.I.), and the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (NRF-2016R1A6A3A03007871 to D.M.). We thank J. L. Robertson at the University of Iowa for sending a plasmid template containing a monomeric ClC-ec1 gene and the members of our lab for comments on the manuscript. Anton 2 computer time was provided by the Pittsburgh Supercomputing Center (PSC) through grant R01GM116961 from the National Institutes of Health. The Anton 2 machine at PSC was generously made available by D. E. Shaw Research.
Author information
Authors and Affiliations
Contributions
D.M., R.E.J. and J.U.B. conceived and designed the experiments. Y.Q. and W.I. designed and performed molecular dynamics simulations. D.M., R.E.J., J.Y.W. and M.A.A. performed plasmid cloning and protein purification. D.M. performed DNA handle conjugation and single-molecule forced unfolding experiments. R.E.J. performed the domain isolation, SEC, CD and vesicle swelling experiments. D.M., R.E.J. and J.U.B. analyzed the experimental data. Y.Q., W.I., D.M. and J.U.B. analyzed the molecular dynamics simulations. D.M., R.E.J., Y.Q., W.I. and J.U.B. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Text and Figures
Supplementary Table 1 and Supplementary Figure 1–10
Supplementary Video 1
Supplementary Video 1
Rights and permissions
About this article
Cite this article
Min, D., Jefferson, R.E., Qi, Y. et al. Unfolding of a ClC chloride transporter retains memory of its evolutionary history. Nat Chem Biol 14, 489–496 (2018). https://doi.org/10.1038/s41589-018-0025-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-018-0025-4