Abstract
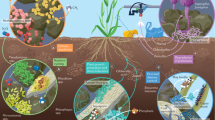
In recent years, there has been considerable progress in determining the soil properties that influence the structure of the soil microbiome. By contrast, the effects of microorganisms on their soil habitat have received less attention with most previous studies focusing on microbial contributions to soil carbon and nitrogen dynamics. However, soil microorganisms are not only involved in nutrient cycling and organic matter transformations but also alter the soil habitat through various biochemical and biophysical mechanisms. Such microbially mediated modifications of soil properties can have local impacts on microbiome assembly with pronounced ecological ramifications. In this Review, we describe the processes by which microorganisms modify the soil environment, considering soil physics, hydrology and chemistry. We explore how microorganism–soil interactions can generate feedback loops and discuss how microbially mediated modifications of soil properties can serve as an alternative avenue for the management and manipulation of microbiomes to combat soil threats and global change.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Whitman, W. B., Coleman, D. C. & Wiebe, W. J. Prokaryotes: the unseen majority. Proc. Natl Acad. Sci. USA 95, 6578–6583 (1998).
Fierer, N. Embracing the unknown: disentangling the complexities of the soil microbiome. Nat. Rev. Microbiol. 15, 579–590 (2017).
Bahram, M. et al. Structure and function of the global topsoil microbiome. Nature 560, 233–237 (2018). This study is a global survey of the soil bacteria and fungi revealing the importance of both environmental filtering and niche differentiation in determining the soil microbial composition.
Keiluweit, M., Wanzek, T., Kleber, M., Nico, P. & Fendorf, S. Anaerobic microsites have an unaccounted role in soil carbon stabilization. Nat. Commun. 8, 1771 (2017).
Schuur, Ea. G. et al. Climate change and the permafrost carbon feedback. Nature 520, 171–179 (2015).
Schimel, J. & Schaeffer, S. Microbial control over carbon cycling in soil. Front. Microbiol. 3, 348 (2012).
Fowler, D. et al. The global nitrogen cycle in the twenty-first century. Philos. Trans. R. Soc. Lond. B Biol. Sci. 368, 20130164 (2013).
Barker, W. W. & Banfield, J. F. Biologically versus inorganically mediated weathering reactions: relationships between minerals and extracellular microbial polymers in lithobiontic communities. Chem. Geol. 132, 55–69 (1996).
Doetterl, S. et al. Links among warming, carbon and microbial dynamics mediated by soil mineral weathering. Nat. Geosci. 11, 589–593 (2018).
Napieralski, S. A. et al. Microbial chemolithotrophy mediates oxidative weathering of granitic bedrock. Proc. Natl Acad. Sci. USA 116, 26394–26401 (2019).
Finlay, R. D. et al. Reviews and syntheses: biological weathering and its consequences at different spatial levels — from nanoscale to global scale. Biogeosciences 17, 1507–1533 (2020).
Sokol, N. W. et al. Life and death in the soil microbiome: how ecological processes influence biogeochemistry. Nat. Rev. Microbiol. 20, 415–430 (2022).
Moreau, D., Bardgett, R. D., Finlay, R. D., Jones, D. L. & Philippot, L. A plant perspective on nitrogen cycling in the rhizosphere. Funct. Ecol. 33, 540–552 (2019).
Schimel, J. P. & Bennett, J. Nitrogen mineralization: challenges of a changing paradigm. Ecology 85, 591–602 (2004).
Geisseler, D., Horwath, W. R., Joergensen, R. G. & Ludwig, B. Pathways of nitrogen utilization by soil microorganisms — a review. Soil Biol. Biochem. 42, 2058–2067 (2010).
Husson, O. Redox potential (Eh) and pH as drivers of soil/plant/microorganism systems: a transdisciplinary overview pointing to integrative opportunities for agronomy. Plant Soil 362, 389–417 (2013).
Bolan, N. S. & Hedley, M. J. Role of carbon, nitrogen, and sulfur cycles in soil acidification. in Handbook of Soil Acidity (CRC Press, 2003).
Sánchez-Cañete, E. P., Barron-Gafford, G. A. & Chorover, J. A considerable fraction of soil-respired CO2 is not emitted directly to the atmosphere. Sci. Rep. 8, 13518 (2018).
Mergelov, N. et al. Alteration of rocks by endolithic organisms is one of the pathways for the beginning of soils on Earth. Sci. Rep. 8, 3367 (2018).
Gadd, G. M. et al. Oxalate production by fungi: significance in geomycology, biodeterioration and bioremediation. Fungal Biol. Rev. 28, 36–55 (2014).
Jones, D. L., Dennis, P. G., Owen, A. G. & van Hees, P. A. W. Organic acid behavior in soils — misconceptions and knowledge gaps. Plant Soil 248, 31–41 (2003).
Hervé, V., Junier, T., Bindschedler, S., Verrecchia, E. & Junier, P. Diversity and ecology of oxalotrophic bacteria. World J. Microbiol. Biotechnol. 32, 28 (2016).
Syed, S., Buddolla, V. & Lian, B. Oxalate carbonate pathway — conversion and fixation of soil carbon — a potential scenario for sustainability. Front. Plant Sci. 11, 591297 (2020).
Norton, J. & Ouyang, Y. Controls and adaptive management of nitrification in agricultural soils. Front. Microbiol. 10, 1931 (2019).
Huet, S. et al. Experimental community coalescence sheds light on microbial interactions in soil and restores impaired functions. Microbiome 11, 42 (2023). This work experimentally demonstrated that depletion of bacterial ammonia oxidizers through community manipulation reduces soil pH.
Fernandes, T. R., Segorbe, D., Prusky, D. & Di Pietro, A. How alkalinization drives fungal pathogenicity. PLoS Pathog. 13, e1006621 (2017).
Palmieri, D., Vitale, S., Lima, G., Di Pietro, A. & Turrà, D. A bacterial endophyte exploits chemotropism of a fungal pathogen for plant colonization. Nat. Commun. 11, 5264 (2020).
Vylkova, S. Environmental pH modulation by pathogenic fungi as a strategy to conquer the host. PLoS Pathog. 13, e1006149 (2017).
Howarth, R. W., Stewart, J. W. B. & Ivanov, M. V. Sulphur Cycling on the Continents: Wetlands, Terrestrial Ecosystems and Associated Water Bodies (John Wiley & Sons, Ltd, 1992).
Koch, T. & Dahl, C. A novel bacterial sulfur oxidation pathway provides a new link between the cycles of organic and inorganic sulfur compounds. ISME J. 12, 2479–2491 (2018).
Yang, Z., Haneklaus, S., Ram Singh, B. & Schnug, E. Effect of repeated applications of elemental sulfur on microbial population, sulfate concentration, and pH in soils. Commun. Soil Sci. Plant Anal. 39, 124–140 (2007).
Linder, T. Assimilation of alternative sulfur sources in fungi. World J. Microbiol. Biotechnol. 34, 51 (2018).
Kappler, A. et al. An evolving view on biogeochemical cycling of iron. Nat. Rev. Microbiol. 19, 360–374 (2021). This study is a comprehensive review of abiotic and biotic processes involved in oxidation of Fe(II) and reduction of Fe(III).
Vaksmaa, A. et al. Stratification of diversity and activity of methanogenic and methanotrophic microorganisms in a nitrogen-fertilized Italian paddy soil. Front. Microbiol. 8, 2127 (2017).
Gabriel, G. V. M. et al. Methane emission suppression in flooded soil from Amazonia. Chemosphere 250, 126263 (2020).
Borch, T. et al. Biogeochemical redox processes and their impact on contaminant dynamics. Environ. Sci. Technol. 44, 15–23 (2010).
Byrne, J. M. et al. Redox cycling of Fe(II) and Fe(III) in magnetite by Fe-metabolizing bacteria. Science 347, 1473–1476 (2015).
Johnstone, T. C. & Nolan, E. M. Beyond iron: non-classical biological functions of bacterial siderophores. Dalton Trans. 44, 6320–6339 (2015).
Schalk, I. J., Hannauer, M. & Braud, A. New roles for bacterial siderophores in metal transport and tolerance. Environ. Microbiol. 13, 2844–2854 (2011).
Gadd, G. M. Metals, minerals and microbes: geomicrobiology and bioremediation. Microbiology 156, 609–643 (2010). 2010.
Samuels, T. et al. in Biogeochemical Cycles 59–79 (American Geophysical Union (AGU), 2020).
Jongmans, A. G. et al. Rock-eating fungi. Nature 389, 682–683 (1997).
Uroz, S., Calvaruso, C., Turpault, M.-P. & Frey-Klett, P. Mineral weathering by bacteria: ecology, actors and mechanisms. Trends Microbiol. 17, 378–387 (2009).
Tang, Y., Zeiner, C. A., Santelli, C. M. & Hansel, C. M. Fungal oxidative dissolution of the Mn(II)-bearing mineral rhodochrosite and the role of metabolites in manganese oxide formation. Environ. Microbiol. 15, 1063–1077 (2013).
Aoki, Y., Hoshino, M. & Matsubara, T. Silica and testate amoebae in a soil under pine–oak forest. Geoderma 142, 29–35 (2007).
Sommer, M. et al. Si cycling in a forest biogeosystem — the importance of transient state biogenic Si pools. Biogeosciences 10, 4991–5007 (2013).
Zhu, T. & Dittrich, M. Carbonate precipitation through microbial activities in natural environment, and their potential in biotechnology: a review. Front. Bioeng. Biotechnol. https://doi.org/10.3389/fbioe.2016.00004 (2016).
Six, J., Bossuyt, H., Degryze, S. & Denef, K. A history of research on the link between (micro)aggregates, soil biota, and soil organic matter dynamics. Soil. Tillage Res. 79, 7–31 (2004).
Chenu, C. & Cosentino, D. Microbial regulation of soil structural dynamics. Archit. Biol. Soils Life Inn. Space https://doi.org/10.1079/9781845935320.0037 (2011).
Ritz, K. & Young, I. M. Interactions between soil structure and fungi. Mycologist 18, 52–59 (2004).
Rillig, M. C. & Mummey, D. L. Mycorrhizas and soil structure. N. Phytol. 171, 41–53 (2006).
Lehmann, A., Zheng, W. & Rillig, M. C. Soil biota contributions to soil aggregation. Nat. Ecol. Evol. 1, 1828–1835 (2017). This study is a comprehensive meta-analysis of the role of soil biota on soil structure, using evidence from experiments across different organism groups.
Totsche, K. U. et al. Microaggregates in soils. J. Plant Nutr. Soil Sci. 181, 104–136 (2018).
Leifheit, E. F., Veresoglou, S. D., Lehmann, A., Morris, E. K. & Rillig, M. C. Multiple factors influence the role of arbuscular mycorrhizal fungi in soil aggregation — a meta-analysis. Plant Soil 374, 523–537 (2014).
Lehmann, A. et al. Fungal traits important for soil aggregation. Front. Microbiol. 10, 2904 (2020).
Guhra, T., Ritschel, T. & Totsche, K. U. Formation of mineral–mineral and organo–mineral composite building units from microaggregate-forming materials including microbially produced extracellular polymeric substances. Eur. J. Soil Sci. 70, 604–615 (2019).
Costa, O. Y. A., Raaijmakers, J. M. & Kuramae, E. E. Microbial extracellular polymeric substances: ecological function and impact on soil aggregation. Front. Microbiol. 9, 1636 (2018).
Chamizo, S., Mugnai, G., Rossi, F., Certini, G. & De Philippis, R. Cyanobacteria inoculation improves soil stability and fertility on different textured soils: gaining insights for applicability in soil restoration. Front. Environ. Sci. https://doi.org/10.3389/fenvs.2018.00049 (2018).
Cuadros, J. Clay minerals interaction with microorganisms: a review. Clay Min. 52, 235–261 (2017).
Krause, L. et al. Initial microaggregate formation: association of microorganisms to montmorillonite-goethite aggregates under wetting and drying cycles. Geoderma 351, 250–260 (2019).
Schimel, J. P. Life in dry soils: effects of drought on soil microbial communities and processes. Annu. Rev. Ecol. Evol. Syst. 49, 409–432 (2018).
Colica, G. et al. Microbial secreted exopolysaccharides affect the hydrological behavior of induced biological soil crusts in desert sandy soils. Soil Biol. Biochem. 68, 62–70 (2014).
Rosenzweig, R., Shavit, U. & Furman, A. Water retention curves of biofilm-affected soils using xanthan as an analogue. Soil Sci. Soc. Am. J. 76, 61–69 (2012).
Chenu, C. Clay- or sand-polysaccharide associations as models for the interface between micro-organisms and soil: water related properties and microstructure. Geoderma 56, 143–156 (1993).
Benard, P. et al. Microhydrological niches in soils: how mucilage and EPS alter the biophysical properties of the rhizosphere and other biological hotspots. Vadose Zone J. 18, 180211 (2019). This work very clearly analyses the physical processes by which root mucilages and EPS alter soil hydrology.
Kroener, E., Zarebanadkouki, M., Kaestner, A. & Carminati, A. Nonequilibrium water dynamics in the rhizosphere: how mucilage affects water flow in soils. Water Resour. Res. 50, 6479–6495 (2014).
Volk, E., Iden, S. C., Furman, A., Durner, W. & Rosenzweig, R. Biofilm effect on soil hydraulic properties: experimental investigation using soil-grown real biofilm: hydraulic properties of biofilm amended soil. Water Resour. Res. 52, 5813–5828 (2016).
Zheng, W. et al. Plant growth-promoting rhizobacteria (PGPR) reduce evaporation and increase soil water retention. Water Resour. Res. 54, 3673–3687 (2018).
Adessi, A., Cruz de Carvalho, R., De Philippis, R., Branquinho, C. & Marques da Silva, J. Microbial extracellular polymeric substances improve water retention in dryland biological soil crusts. Soil Biol. Biochem. 116, 67–69 (2018).
Or, D., Phutane, S. & Dechesne, A. Extracellular polymeric substances affecting pore-scale hydrologic conditions for bacterial activity in unsaturated soils. Vadose Zone J. 6, 298–305 (2007).
Morales, V. L., Parlange, J.-Y. & Steenhuis, T. S. Are preferential flow paths perpetuated by microbial activity in the soil matrix? A review. J. Hydrol. 393, 29–36 (2010).
Chenu, C. & Roberson, E. B. Diffusion of glucose in microbial extracellular polysaccharide as affected by water potential. Soil Biol. Biochem. 28, 877–884 (1996).
Guo, Y.-S. et al. Bacterial extracellular polymeric substances amplify water content variability at the pore scale. Front. Environ. Sci. https://doi.org/10.3389/fenvs.2018.00093 (2018).
Deng, J. Z. et al. Synergistic effects of soil microstructure and bacterial EPS on drying rate in emulated soil micromodels. Soil. Biol. Amp Biochem. 83, 116–124 (2015).
Buchmann, C., Steinmetz, Z., Brax, M., Peth, S. & Schaumann, G. E. Effect of matric potential and soil–water–hydrogel interactions on biohydrogel-induced soil microstructural stability. Geoderma 362, 114142 (2020).
Rillig, M. C. A connection between fungal hydrophobins and soil water repellency? Pedobiologia 49, 395–399 (2005).
Rabot, E., Wiesmeier, M., Schlüter, S. & Vogel, H.-J. Soil structure as an indicator of soil functions: a review. Geoderma 314, 122–137 (2018).
Feeney, D. S. et al. Three-dimensional microorganization of the soil–root–microbe system. Microb. Ecol. 52, 151–158 (2006).
Helliwell, J. R., Miller, A. J., Whalley, W. R., Mooney, S. J. & Sturrock, C. J. Quantifying the impact of microbes on soil structural development and behaviour in wet soils. Soil Biol. Biochem. 74, 138–147 (2014).
De Gryze, S. et al. Pore structure changes during decomposition of fresh residue: X-ray tomography analyses. Geoderma 134, 82–96 (2006).
Zhang, W., Gao, W., Whalley, W. R. & Ren, T. Physical properties of a sandy soil as affected by incubation with a synthetic root exudate: strength, thermal and hydraulic conductivity, and evaporation. Eur. J. Soil Sci. 72, 782–792 (2021).
Egerton-Warburton, L. M., Querejeta, J. I. & Allen, M. F. Common mycorrhizal networks provide a potential pathway for the transfer of hydraulically lifted water between plants. J. Exp. Bot. 58, 1473–1483 (2007).
Allen, M. F. Mycorrhizal fungi: highways for water and nutrients in arid soils. Vadose Zone J. 6, 291–297 (2007).
Jia, Y., van der Heijden, M. G. A., Wagg, C., Feng, G. & Walder, F. Symbiotic soil fungi enhance resistance and resilience of an experimental grassland to drought and nitrogen deposition. J. Ecol. 109, 3171–3181 (2021).
Singh, D., Mathimaran, N., Boller, T. & Kahmen, A. Bioirrigation: a common mycorrhizal network facilitates the water transfer from deep-rooted pigeon pea to shallow-rooted finger millet under drought. Plant Soil 440, 277–292 (2019).
Bahadur, A. et al. Mechanistic insights into arbuscular mycorrhizal fungi-mediated drought stress tolerance in plants. Int. J. Mol. Sci. 20, 4199 (2019).
Kakouridis, A. et al. Routes to roots: direct evidence of water transport by arbuscular mycorrhizal fungi to host plants. N. Phytol. 236, 210–221 (2022).
Bach, E. M., Williams, R. J., Hargreaves, S. K., Yang, F. & Hofmockel, K. S. Greatest soil microbial diversity found in micro-habitats. Soil Biol. Biochem. 118, 217–226 (2018).
Odling-Smee, J., Erwin, D. H., Palkovacs, E. P., Feldman, M. W. & Laland, K. N. Niche construction theory: a practical guide for ecologists. Q. Rev. Biol. 88, 3–28 (2013).
Matthews, B. et al. Under niche construction: an operational bridge between ecology, evolution, and ecosystem science. Ecol. Monogr. 84, 245–263 (2014).
Roman, M. S. & Wagner, A. An enormous potential for niche construction through bacterial cross-feeding in a homogeneous environment. PLoS Comput. Biol. 14, e1006340 (2018).
Callahan, B. J., Fukami, T. & Fisher, D. S. Rapid evolution of adaptive niche construction in experimental microbial populations. Evolution 68, 3307–3316 (2014).
Poulton, S. W. et al. A 200-million-year delay in permanent atmospheric oxygenation. Nature 592, 232–236 (2021).
Olejarz, J., Iwasa, Y., Knoll, A. H. & Nowak, M. A. The great oxygenation event as a consequence of ecological dynamics modulated by planetary change. Nat. Commun. 12, 3985 (2021).
Pausas, J. G. & Bond, W. J. Feedbacks in ecology and evolution. Trends Ecol. Evol. 37, 637–644 (2022).
Laland, K. N. & O’Brien, M. J. Niche construction theory and archaeology. J. Archeol. Meth. Theory 17, 303–322 (2010).
Kennedy, M., Droser, M., Mayer, L. M., Pevear, D. & Mrofka, D. Late Precambrian oxygenation; inception of the clay mineral factory. Science 311, 1446–1449 (2006).
Hazen, R. M. Evolution of minerals. Sci. Am. 302, 58–65 (2010).
Hemkemeyer, M. et al. Artificial soil studies reveal domain-specific preferences of microorganisms for the colonisation of different soil minerals and particle size fractions. FEMS Microbiol. Ecol. 90, 770–782 (2014).
Whitman, T. et al. Microbial community assembly differs across minerals in a rhizosphere microcosm. Environ. Microbiol. 20, 4444–4460 (2018).
Heckman, K. et al. The influence of goethite and gibbsite on soluble nutrient dynamics and microbial community composition. Biogeochemistry 112, 179–195 (2013).
Lentini, C., Wankel, S. & Hansel, C. Enriched iron(III)-reducing bacterial communities are shaped by carbon substrate and iron oxide mineralogy. Front. Microbiol. 3, 404 (2012).
Uroz, S., Kelly, L. C., Turpault, M.-P., Lepleux, C. & Frey-Klett, P. The mineralosphere concept: mineralogical control of the distribution and function of mineral-associated bacterial communities. Trends Microbiol. 23, 751–762 (2015).
Nicolitch, O. et al. A microcosm approach highlights the response of soil mineral weathering bacterial communities to an increase of K and Mg availability. Sci. Rep. 9, 14403 (2019).
Sun, Q., Fu, Z., Finlay, R. & Lian, B. Transcriptome analysis provides novel insights into the capacity of the ectomycorrhizal fungus Amanita pantherina to weather k-containing feldspar and apatite. Appl. Environ. Microbiol. 85, e00719 (2019).
Primo, E. et al. Exopolysaccharide II is relevant for the survival of Sinorhizobium meliloti under water deficiency and salinity stress. Molecules 25, 4876 (2020).
Upadhyay, S. K., Singh, J. S. & Singh, D. P. Exopolysaccharide-producing plant growth-promoting rhizobacteria under salinity condition. Pedosphere 21, 214–222 (2011).
Roberson, E. B. & Firestone, M. K. Relationship between desiccation and exopolysaccharide production in a soil Pseudomonas sp. Appl. Environ. Microbiol. 58, 1284–1291 (1992).
Ratzke, C., Denk, J. & Gore, J. Ecological suicide in microbes. Nat. Ecol. Evol. 2, 867–872 (2018).
Romdhane, S. et al. Unraveling negative biotic interactions determining soil microbial community assembly and functioning. ISME J. 16, 296–306 (2021).
Philippot, L. et al. Dissimilatory nitrite-reductase provides a competitive advantage to Pseudomonas sp. RTC01 to colonise the centre of soil aggregates. FEMS Microbiol. Ecol. 21, 175–185 (1996).
Schlüter, S. et al. Denitrification in soil aggregate analogues-effect of aggregate size and oxygen diffusion. Front. Environ. Sci. https://doi.org/10.3389/fenvs.2018.00017 (2018).
Sexstone, A. J., Revsbech, N. P., Parkin, T. B. & Tiedje, J. M. Direct measurement of oxygen profiles and denitrification rates in soil aggregates. Soil Sci. Soc. Am. J. 49, 645–651 (1985). This work describes the use of microelectrodes to demonstrate how O2 profiles and the presence of anoxic microenvironments in soil aggregates determine the potential for microbial denitrification.
Loudon, C. M., Matthews, B., Sevilgen, D. S. & Ibelings, B. W. Experimental evidence that evolution by niche construction affects dissipative ecosystem dynamics. Evol. Ecol. 30, 221–234 (2016).
Yuste, J. C. et al. Drought-resistant fungi control soil organic matter decomposition and its response to temperature. Glob. Change Biol. 17, 1475–1486 (2011).
de Vries, F. T. et al. Soil bacterial networks are less stable under drought than fungal networks. Nat. Commun. 9, 3033 (2018).
Liu, B. et al. Bio-remediation of desiccation cracking in clayey soils through microbially induced calcite precipitation (MICP). Eng. Geol. 264, 105389 (2020).
Wösten, H. A. B. Hydrophobins: multipurpose proteins. Annu. Rev. Microbiol. 55, 625–646 (2001).
Schiessl, K. T., Janssen, E. M.-L., Kraemer, S. M., McNeill, K. & Ackermann, M. Magnitude and mechanism of siderophore-mediated competition at low iron solubility in the Pseudomonas aeruginosa pyochelin system. Front. Microbiol. 8, 1964 (2017).
Niehus, R., Picot, A., Oliveira, N. M., Mitri, S. & Foster, K. R. The evolution of siderophore production as a competitive trait: the competitive evolution of siderophores. Evolution 71, 1443–1455 (2017).
Hesse, E. et al. Ecological selection of siderophore‐producing microbial taxa in response to heavy metal contamination. Ecol. Lett. 21, 117–127 (2018).
Sexton, D. J. & Schuster, M. Nutrient limitation determines the fitness of cheaters in bacterial siderophore cooperation. Nat. Commun. 8, 230 (2017).
Butaitė, E., Baumgartner, M., Wyder, S. & Kümmerli, R. Siderophore cheating and cheating resistance shape competition for iron in soil and freshwater Pseudomonas communities. Nat. Commun. 8, 414 (2017).
Rodriguez-Caballero, E. et al. Dryland photoautotrophic soil surface communities endangered by global change. Nat. Geosci. 11, 185–189 (2018).
Campbell, S. E. Soil stabilization by a prokaryotic desert crust: implications for Precambrian land biota. Orig. Life 9, 335–348 (1979).
Belnap, J. & Gardner, J. S. Soil microstructure in soils of the Colorado Plateau: the role of the cyanobacterium Microcoleus vaginatus. Gt. Basin Nat. 53, 40–47 (1993).
Miralles, I., Soria, R., Lucas-Borja, M. E., Soriano, M. & Ortega, R. Effect of biocrusts on bacterial community composition at different soil depths in Mediterranean semi-arid ecosystems. Sci. Total Environ. 733, 138613 (2020).
Swenson, T. L., Karaoz, U., Swenson, J. M., Bowen, B. P. & Northen, T. R. Linking soil biology and chemistry in biological soil crust using isolate exometabolomics. Nat. Commun. 9, 19 (2018).
Chamizo, S., Cantón, Y., Miralles-Mellado, I. & Domingo, F. Biological soil crust development affects physicochemical characteristics of soil surface in semiarid ecosystems. Soil Biol. Biochem. 49, 96–105 (2012).
Couradeau, E., Giraldo-Silva, A., De Martini, F. & Garcia-Pichel, F. Spatial segregation of the biological soil crust microbiome around its foundational cyanobacterium, Microcoleus vaginatus, and the formation of a nitrogen-fixing cyanosphere. Microbiome 7, 55 (2019).
Lan, S., Wu, L., Adessi, A. & Hu, C. Cyanobacterial persistence and influence on microbial community dynamics over 15 years in induced biocrusts. Environ. Microbiol. 24, 66–81 (2022).
Vos, M., Wolf, A. B., Jennings, S. J. & Kowalchuk, G. A. Micro-scale determinants of bacterial diversity in soil. FEMS Microbiol. Rev. 37, 936–954 (2013).
Védère, C., Vieublé Gonod, L., Nunan, N. & Chenu, C. Opportunities and limits in imaging microorganisms and their activities in soil microhabitats. Soil Biol. Biochem. 174, 108807 (2022).
Geisseler, D. & Scow, K. M. Long-term effects of mineral fertilizers on soil microorganisms — a review. Soil Biol. Biochem. 75, 54–63 (2014).
Johnsen, K., Jacobsen, C. S., Torsvik, V. & Sørensen, J. Pesticide effects on bacterial diversity in agricultural soils — a review. Biol. Fertil. Soils 33, 443–453 (2001).
Cania, B. et al. Site-specific conditions change the response of bacterial producers of soil structure-stabilizing agents such as exopolysaccharides and lipopolysaccharides to tillage intensity. Front. Microbiol. 11, 568 (2020).
Sae-Tun, O. et al. Fungal biomass and microbial necromass facilitate soil carbon sequestration and aggregate stability under different soil tillage intensities. Appl. Soil. Ecol. 179, 104599 (2022).
Lindstrom, M., Lobb, D. & Schumacher, T. Tillage erosion: an overview. Ann. Arid. Zone 40, 337–350 (2001).
Lal, R., Reicosky, D. C. & Hanson, J. D. Evolution of the plow over 10,000 years and the rationale for no-till farming. Soil. Tillage Res. 93, 1–12 (2007).
Zhao, Z.-B. et al. Fertilization changes soil microbiome functioning, especially phagotrophic protists. Soil Biol. Biochem. 148, 107863 (2020).
Hallin, S., Jones, C. M., Schloter, M. & Philippot, L. Relationship between N-cycling communities and ecosystem functioning in a 50-year-old fertilization experiment. ISME J. 3, 597–605 (2009).
Cederlund, H. et al. Soil carbon quality and nitrogen fertilization structure bacterial communities with predictable responses of major bacterial phyla. Appl. Soil Ecol. 84, 62–68 (2014).
Beeckman, F., Motte, H. & Beeckman, T. Nitrification in agricultural soils: impact, actors and mitigation. Curr. Opin. Biotechnol. 50, 166–173 (2018).
Zhu, X., Burger, M., Doane, T. A. & Horwath, W. R. Ammonia oxidation pathways and nitrifier denitrification are significant sources of N2O and NO under low oxygen availability. Proc. Natl Acad. Sci. USA 110, 6328–6333 (2013).
Lu, L. et al. Nitrification of archaeal ammonia oxidizers in acid soils is supported by hydrolysis of urea. ISME J. 6, 1978–1984 (2012).
Hinckley, E.-L. S., Crawford, J. T., Fakhraei, H. & Driscoll, C. T. A shift in sulfur-cycle manipulation from atmospheric emissions to agricultural additions. Nat. Geosci. 13, 597–604 (2020).
Zhao, C., Degryse, F., Gupta, V. & McLaughlin, M. J. Elemental sulfur oxidation in Australian cropping soils. Soil Sci. Soc. Am. J. 79, 89–96 (2015).
Crouzet, O. et al. Soil photosynthetic microbial communities mediate aggregate stability: influence of cropping systems and herbicide use in an agricultural soil. Front. Microbiol. 10, 1319 (2019).
Krashevska, V., Klarner, B., Widyastuti, R., Maraun, M. & Scheu, S. Changes in structure and functioning of protist (Testate amoebae) communities due to conversion of lowland rainforest into rubber and oil palm plantations. PLoS ONE 11, e0160179 (2016).
Philippot, L., Raaijmakers, J. M., Lemanceau, P. & van der Putten, W. H. Going back to the roots: the microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 11, 789–799 (2013).
Sher, Y. et al. Microbial extracellular polysaccharide production and aggregate stability controlled by switchgrass (Panicum virgatum) root biomass and soil water potential. Soil. Biol. Biochem. 143, 107742 (2020).
Whiffin, V. S., van Paassen, L. A. & Harkes, M. P. Microbial carbonate precipitation as a soil improvement technique. Geomicrobiol. J. 24, 417–423 (2007).
Naveed, M. et al. Application of microbially induced calcium carbonate precipitation with urea hydrolysis to improve the mechanical properties of soil. Ecol. Eng. 153, 105885 (2020).
DeJong, J. T. et al. Soil engineering in vivo: harnessing natural biogeochemical systems for sustainable, multi-functional engineering solutions. J. R. Soc. Interface 8, 1–15 (2011).
Dejong, J. T. et al. Biogeochemical processes and geotechnical applications: progress, opportunities and challenges. Géotechnique 63, 287–301 (2013).
Raveh-Amit, H. & Tsesarsky, M. Biostimulation in desert soils for microbial-induced calcite precipitation. Appl. Sci. 10, 2905 (2020).
Kiasari, M., Pakbaz, M. & Ghezelbash, G. Increasing of soil urease activity by stimulation of indigenous bacteria and investigation of their role on shear strength. Geomicrobiol. J. 35, 1–8 (2018).
Gowthaman, S., Iki, T., Nakashima, K., Ebina, K. & Kawasaki, S. Feasibility study for slope soil stabilization by microbial induced carbonate precipitation (MICP) using indigenous bacteria isolated from cold subarctic region. SN Appl. Sci. 1, 1480 (2019).
Salifu, E., MacLachlan, E., Iyer, K. R., Knapp, C. W. & Tarantino, A. Application of microbially induced calcite precipitation in erosion mitigation and stabilisation of sandy soil foreshore slopes: a preliminary investigation. Eng. Geol. 201, 96–105 (2016).
Meng, H., Gao, Y., He, J., Qi, Y. & Hang, L. Microbially induced carbonate precipitation for wind erosion control of desert soil: field-scale tests. Geoderma 383, 114723 (2021).
Liu, B. et al. Potential drought mitigation through microbial induced calcite precipitation-MICP. Water Resour. Res. 57, e2020WR029434 (2021).
Chu, J., Stabnikov, V. & Ivanov, V. Microbially induced calcium carbonate precipitation on surface or in the bulk of soil. Geomicrobiol. J. 29, 544–549 (2012).
Park, C.-H., Li, X. R., Zhao, Y., Jia, R. L. & Hur, J.-S. Rapid development of cyanobacterial crust in the field for combating desertification. PLoS ONE 12, e0179903 (2017).
Malam Issa, O. et al. Effects of the inoculation of cyanobacteria on the microstructure and the structural stability of a tropical soil. Plant Soil 290, 209–219 (2007).
Chamizo, S., Adessi, A., Certini, G. & De Philippis, R. Cyanobacteria inoculation as a potential tool for stabilization of burned soils. Restor. Ecol. 28, S106–S114 (2020).
Sadeghi, S. H., Sadeghi Satri, M., Kheirfam, H. & Zarei Darki, B. Runoff and soil loss from small plots of erosion-prone marl soil inoculated with bacteria and cyanobacteria under real conditions. Eur. J. Soil Biol. 101, 103214 (2020).
Perruchon, C. et al. Characterization of the biodegradation, bioremediation and detoxification capacity of a bacterial consortium able to degrade the fungicide thiabendazole. Biodegradation 28, 383–394 (2017).
Billet, L., Devers, M., Rouard, N., Martin-Laurent, F. & Spor, A. Labour sharing promotes coexistence in atrazine degrading bacterial communities. Sci. Rep. 9, 18363 (2019).
Meyer, L., Guyot, S., Chalot, M. & Capelli, N. The potential of microorgansims as biomonitoring and bioremediation tools for mercury-contaminated soils. Ecotox. Env. Saf. 262, 115185 (2023).
Zhang, H., Yuan, X., Xiong, T., Wang, H. & Jiang, L. Bioremediation of co-contaminated soil with heavy metals and pesticides: influence factors, mechanisms and evaluation methods. Chem. Eng. J. 398, 125657 (2020).
Arantza, S.-J. et al. Bio- and phytoremediation: plants and microbes to the rescue of heavy metal polluted soils. SN Appl. Sci. 4, 59 (2022).
Xue, Z.-F., Cheng, W.-C., Wang, L. & Hu, W. Effects of bacterial inoculation and calcium source on microbial-induced carbonate precipitation for lead remediation. J. Hazard. Mater. 426, 128090 (2022).
Cuaxinque-Flores, G. et al. Bioimmobilization of toxic metals by precipitation of carbonates using Sporosarcina luteola: an in vitro study and application to sulfide-bearing tailings. Sci. Total Environ. 724, 138124 (2020).
Falkowski, P. G., Fenchel, T. & Delong, E. F. The microbial engines that drive earth’s biogeochemical cycles. Science 320, 1034–1039 (2008).
Ribeiro, I. D. A. et al. Use of mineral weathering bacteria to enhance nutrient availability in crops: a review. Front. Plant. Sci. 11, 590774 (2020).
Pramanik, P., Goswami, A. J., Gosh, S. & Kalita, C. An indigenous strain of potassium‐solubilizing bacteria Bacillus pseudomycoides enhanced potassium uptake in tea plants by increasing potassium availability in the mica waste‐treated soil of North‐east India. J. Appl. Microbiol. 126, 215–222 (2019).
EUROPA. IPCC Fifth Assessment Report: Climate Change 2014 (AR5). European Environment Agency https://www.eea.europa.eu/data-and-maps/indicators/greenhouse-gas-emission-trends-5/ipcc-fifth-assessment-report-climate (2014).
McCutcheon, J., Wilson, S. A. & Southam, G. Microbially accelerated carbonate mineral precipitation as a strategy for in situ carbon sequestration and rehabilitation of asbestos mine sites. Environ. Sci. Technol. 50, 1419–1427 (2016).
Rodell, M. & Li, B. Changing intensity of hydroclimatic extreme events revealed by GRACE and GRACE-FO. Nat. Water 1, 241–248 (2023).
Roper, M. M. Potential for remediation of water repellent soils by inoculation with wax-degrading bacteria in south-western Australia. Biologia 61, S358–S362 (2006).
Lowe, M.-A. et al. Bacillus subtilis and surfactant amendments for the breakdown of soil water repellency in a sandy soil. Geoderma 344, 108–118 (2019).
Batista, B. D. & Singh, B. K. Realities and hopes in the application of microbial tools in agriculture. Microb. Biotechnol. 14, 1258–1268 (2021).
Kaminsky, L. M., Trexler, R. V., Malik, R. J., Hockett, K. L. & Bell, T. H. The inherent conflicts in developing soil microbial inoculants. Trends Biotechnol. 37, 140–151 (2019).
Jack, C. N., Petipas, R. H., Cheeke, T. E., Rowland, J. L. & Friesen, M. L. Microbial inoculants: silver bullet or microbial jurassic park? Trends Microbiol. 29, 299–308 (2021).
Liu, X., Le Roux, X. & Salles, J. F. The legacy of microbial inoculants in agroecosystems and potential for tackling climate change challenges. iScience 25, 103821 (2022).
Santos, M. S., Nogueira, M. A. & Hungria, M. Microbial inoculants: reviewing the past, discussing the present and previewing an outstanding future for the use of beneficial bacteria in agriculture. AMB Express 9, 205 (2019).
Waltz, E. A new crop of microbe startups raises big bucks, takes on the establishment. Nat. Biotechnol. 35, 1120–1122 (2017).
Mitchell, A. C. & Ferris, F. G. The influence of Bacillus pasteurii on the nucleation and growth of calcium carbonate. Geomicrobiol. J. 23, 213–226 (2006).
Wen, K., Li, L., Zhang, R., Li, Y. & Amini, F. Micro-scale analysis of microbial-induced calcite precipitation in sandy soil through SEM/FIB imaging. Microsc. Today 27, 24–29 (2019).
Lehmann, A., Leifheit, E. F. & Rillig, M. C. in Mycorrhizal Mediation of Soil (eds Johnson, N. C., Gehring, C. & Jansa, J.) Ch. 14, 241–262 (Elsevier, 2017).
Dorioz, J. M. D., Robert, M. & Chenu, C. The role of roots, fungi and bacteria on clay particle organization. An experimental approach. Geoderma 56, 179–194 (1993).
Angers, D. & Chenu, C. in Soil Processes and the Carbon Cycle 199–206 (CRC Press, 1997).
Crowther, T. W. et al. The global soil community and its influence on biogeochemistry. Science 365, eaav0550 (2019).
Jansson, J. K. & Hofmockel, K. S. Soil microbiomes and climate change. Nat. Rev. Microbiol. 18, 35–46 (2020).
Lv, J., Huang, Z., Luo, L., Zhang, S. & Wang, Y. Advances in molecular and microscale characterization of soil organic matter: current limitations and future prospects. Environ. Sci. Technol. 56, 12793–12810 (2022).
Islam, W., Noman, A., Naveed, H., Huang, Z. & Chen, H. Y. H. Role of environmental factors in shaping the soil microbiome. Environ. Sci. Pollut. Res. 27, 41225–41247 (2020).
Hinsinger, P., Plassard, C., Tang, C. & Jaillard, B. Origins of root-mediated pH changes in the rhizosphere and their responses to environmental constraints: a review. Plant Soil 248, 43–59 (2003).
Lurthy, T. et al. Impact of bacterial siderophores on iron status and ionome in pea. Front. Plant Sci. 11, 730 (2020).
Bar-Ness, E., Hadar, Y., Chen, Y., Shanzer, A. & Libman, J. Iron uptake by plants from microbial siderophores. Plant Physiol. 99, 1329–1335 (1992).
Maisch, M., Lueder, U., Kappler, A. & Schmidt, C. From plant to paddy — how rice root iron plaque can affect the paddy field iron cycling. Soil Syst. 4, 28 (2020).
Smits, M. M., Bonneville, S., Benning, L. G., Banwart, S. A. & Leake, J. R. Plant-driven weathering of apatite — the role of an ectomycorrhizal fungus. Geobiology 10, 445–456 (2012).
Mujinya, B. B. et al. The origin of carbonates in termite mounds of the Lubumbashi area, D.R. Congo. Geoderma 165, 95–105 (2011).
Cailleau, G., Braissant, O. & Verrecchia, E. Turning sunlight into stone: the oxalate–carbonate pathway in a tropical tree ecosystem. Biogeosciences 8, 1755–1767 (2011).
Geisen, S. et al. Soil protists: a fertile frontier in soil biology research. FEMS Microbiol. Rev. 42, 293–323 (2018). This paper provides an outlook for research on soil protists, serving as an entry point for researchers interested in this organism group and its effects in soil.
Erktan, A., Rillig, M. C., Carminati, A., Jousset, A. & Scheu, S. Protists and collembolans alter microbial community composition, C dynamics and soil aggregation in simplified consumer–prey systems. Biogeosciences 17, 4961–4980 (2020). This study is a comprehensive analysis of the complex interactions among soil structure, soil organic matter and soil organisms within trophic networks.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Microbiology thanks Samiran Banerjee and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Adaptative radiation
-
The rapid diversification of species as a consequence of adaption to different environmental conditions.
- Ammonification
-
The respiratory reduction of nitrate to ammonium when oxygen is limiting.
- Arbuscular mycorrhizal fungi
-
Fungi that form a symbiosis with plants by penetrating the cortical cells of the roots of a vascular plant.
- Denitrification
-
The respiratory reduction of nitrogen oxides to N2O and N2 when oxygen is limiting.
- Extracellular polymeric substances
-
(EPSs). Polymeric organic compounds (mainly polysaccharides, proteins and nucleic acids) that are produced and released by microorganisms.
- Hydrophobins
-
Small proteins produced by filamentous fungi that can spontaneously self-assemble and change the polarity of a surface.
- Niche construction theory
-
The concept that organisms can modify their environment and that, in turn, these changes influence the organisms.
- Nitrification
-
Aerobic oxidation of ammonium to nitrite and then to nitrate to generate energy.
- Photosynthetic autotrophs
-
An organism that uses light energy to fix CO2.
- Siderophores
-
Organic compounds that are produced and released by microorganisms to make otherwise poorly soluble Fe(III) ions bioavailable for the cells and to facilitate their uptake.
- Soil micropores
-
Pores in which water is essentially held by capillary forces (≤0.08 mm), nearly immobile and in which solute movement is limited to diffusion.
- Weathering
-
The process of breaking down or dissolving solids (minerals and rocks) by biological, chemical or physical processes.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Philippot, L., Chenu, C., Kappler, A. et al. The interplay between microbial communities and soil properties. Nat Rev Microbiol 22, 226–239 (2024). https://doi.org/10.1038/s41579-023-00980-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41579-023-00980-5
This article is cited by
-
Interplay of biotic and abiotic factors shapes tree seedling growth and root-associated microbial communities
Communications Biology (2024)
-
Microbes in porous environments: from active interactions to emergent feedback
Biophysical Reviews (2024)
-
Heterogeneity in Arbuscular Mycorrhizal Fungi and Plant Communities of the Brazilian Cerrado, Transitional Areas toward the Caatinga, and the Atlantic Forest
Microbial Ecology (2024)