Abstract
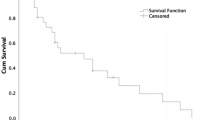
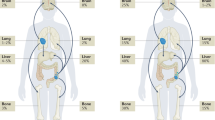
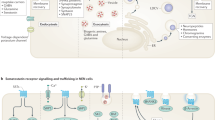
Gastric neuroendocrine neoplasms (gNENs) display peculiar site-specific features among all NENs. Their incidence and prevalence have been rising in the past few decades. gNENs comprise gastric neuroendocrine carcinomas (gNECs) and gastric neuroendocrine tumours (gNETs), the latter further classified into three types. Type I anatype II gNETs are gastrin-dependent and develop in chronic atrophic gastritis and as part of Zollinger–Ellison syndrome within a multiple endocrine neoplasia type 1 syndrome (MEN1), respectively. Type III or sporadic gNETs develop in the absence of hypergastrinaemia and in the context of a near-normal or inflamed gastric mucosa. gNECs can also develop in the context of variable atrophic, relatively normal or inflamed gastric mucosa. Each gNEN type has different clinical characteristics and requires a different multidisciplinary approach in expert dedicated centres. Type I gNETs are managed mainly by endoscopy or surgery, whereas the treatment of type II gNETs largely depends on the management of the concomitant MEN1. Type III gNETs may require both locoregional approaches and systemic treatments; NECs are often metastatic and therefore require systemic treatment. Specific data regarding the systemic treatment of gNENs are lacking and are derived from the treatment of intestinal NETs and NECs. An enhanced understanding of molecular and clinical pathophysiology is needed to improve the management and outcomes of patients’ gNETs.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
WHO Classification of Tumours Editorial Board. WHO Classification of Endocrine and Neuroendocrine Tumours (WHO, 2022).
WHO Classification of Tumours Editorial Board. WHO Classification of Digestive Tumours (WHO, 2019).
Rindi, G. et al. Overview of the 2022 WHO Classification of Neuroendocrine Neoplasms. Endocr. Pathol. 33, 115–154 (2022).
Raj, N. et al. Real-time genomic characterization of metastatic pancreatic neuroendocrine tumors has prognostic implications and identifies potential germline actionability. JCO Precis. Oncol. 2018, PO.17.00267 (2018).
Rindi, G., Luinetti, O., Cornaggia, M., Capella, C. & Solcia, E. Three subtypes of gastric argyrophil carcinoid and the gastric neuroendocrine carcinoma: a clinicopathologic study. Gastroenterology 104, 994–1006 (1993).
Rindi, G. et al. Gastric carcinoids and neuroendocrine carcinomas: pathogenesis, pathology, and behavior. World J. Surg. 20, 168–172 (1996).
Rindi, G. et al. ECL cell tumor and poorly differentiated endocrine carcinoma of the stomach: prognostic evaluation by pathological analysis. Gastroenterology 116, 532–542 (1999).
Vanoli, A. et al. Prognostic evaluations tailored to specific gastric neuroendocrine neoplasms: analysis of 200 cases with extended follow-up. Neuroendocrinology 107, 114–126 (2018).
Dasari, A. et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 3, 1335–1342 (2017). This is the largest population-based study report to date to highlight epidemiology of neuroendocrine tumours, including those of the stomach.
Rustgi, S. D. et al. Epidemiology of gastric malignancies 2000–2018 according to histology: a population-based analysis of incidence and temporal trends. Clin. Gastroenterol. Hepatol. 21, 3285–3295.e8 (2023).
IARC. Cancer Today stomach cancer data. WHO https://gco.iarc.fr/today/en/dataviz/tables?mode=population&cancers=7 (2022).
Ellis, L., Shale, M. J. & Coleman, M. P. Carcinoid tumors of the gastrointestinal tract: trends in incidence in England since 1971. Am. J. Gastroenterol. 105, 2563–2569 (2010).
Carmack, S. W., Genta, R. M., Schuler, C. M. & Saboorian, M. H. The current spectrum of gastric polyps: a 1-year national study of over 120,000 patients. Am. J. Gastroenterol. 104, 1524–1532 (2009).
Vannella, L. et al. Development of type I gastric carcinoid in patients with chronic atrophic gastritis. Aliment. Pharmacol. Ther. 33, 1361–1369 (2011).
Shah, S. C., Piazuelo, M. B., Kuipers, E. J. & Li, D. AGA clinical practice update on the diagnosis and management of atrophic gastritis: expert review. Gastroenterology 161, 1325–1332.e7 (2021).
Garcia-Carbonero, R. et al. Incidence, patterns of care and prognostic factors for outcome of gastroenteropancreatic neuroendocrine tumors (GEP-NETs): results from the National Cancer Registry of Spain (RGETNE). Ann. Oncol. 21, 1794–1803 (2010).
Genus, T. S. E. et al. Impact of neuroendocrine morphology on cancer outcomes and stage at diagnosis: a UK nationwide cohort study 2013–2015. Br. J. Cancer 121, 966–972 (2019).
Palepu, J. et al. Trends in diagnosis of gastroenteropancreatic neuroendocrine tumors (GEP-NETs) in India: a report of multicenter data from a web-based registry. Indian. J. Gastroenterol. 36, 445–451 (2017).
Fan, J.-H. et al. A nation-wide retrospective epidemiological study of gastroenteropancreatic neuroendocrine neoplasms in china. Oncotarget 8, 71699–71708 (2017).
Tsai, H.-J. et al. The epidemiology of neuroendocrine tumors in Taiwan: a nation-wide cancer registry-based study. PLoS ONE 8, e62487 (2013).
Masui, T., Ito, T., Komoto, I. & Uemoto, S. Recent epidemiology of patients with gastro-entero-pancreatic neuroendocrine neoplasms (GEP-NEN) in Japan: a population-based study. BMC Cancer 20, 1104 (2020).
Eom, B. W., Jung, K.-W., Won, Y.-J., Yang, H. & Kim, Y.-W. Trends in gastric cancer incidence according to the clinicopathological characteristics in Korea, 1999–2014. Cancer Res. Treat. 50, 1343–1350 (2018).
Lahner, E. et al. Gastric cancer in patients with type I gastric carcinoids. Gastric Cancer 18, 564–570 (2015).
Lenti, M. V. et al. Autoimmune gastritis. Nat. Rev. Dis. Prim. 6, 56 (2020).
Vannella, L., Lahner, E., Osborn, J. & Annibale, B. Systematic review: gastric cancer incidence in pernicious anaemia. Aliment. Pharmacol. Ther. 37, 375–382 (2013).
Miceli, E. et al. Long-term natural history of autoimmune gastritis: results from a prospective, monocentric series. Am. J. Gastroenterol. https://doi.org/10.14309/ajg.0000000000002619 (2023).
Panzuto, F. et al. European Neuroendocrine Tumor Society (ENETS) 2023 guidance paper for gastroduodenal neuroendocrine tumours (NETs) G1–G3. J. Neuroendocrinol. 35, e13306 (2023). This paper discusses the guidelines of the European Neuroendocrine Tumor Society (ENETS) regarding the clinical management of gastric neuroendocrine tumours.
Yao, J. C. et al. One hundred years after ‘carcinoid’: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J. Clin. Oncol. 26, 3063–3072 (2008).
Panzuto, F. et al. Tumour type and size are prognostic factors in gastric neuroendocrine neoplasia: a multicentre retrospective study. Dig. Liver Dis. 51, 1456–1460 (2019). This is a large multicentre retrospective study to evaluate prognostic factors in gastric neuroendocrine tumours.
Felder, S. et al. Gastric neuroendocrine neoplasias: manifestations and comparative outcomes. Endocr. Relat. Cancer 26, 751–763 (2019).
Exarchou, K., Howes, N. & Pritchard, D. M. Systematic review: management of localised low‐grade upper gastrointestinal neuroendocrine tumours. Aliment. Pharmacol. Ther. 51, 1247–1267 (2020).
Laffi, A. et al. Gastric neuroendocrine tumors (g-NETs): a systematic review of the management and outcomes of type 3 g-NETs. Cancers 15, 2202 (2023).
Min, B.-H. et al. Clinicopathological features and outcome of type 3 gastric neuroendocrine tumours. Br. J. Surg. 105, 1480–1486 (2018).
Engevik, A. C., Kaji, I. & Goldenring, J. R. The physiology of the gastric parietal cell. Physiol. Rev. 100, 573–602 (2020). This is a paper on the state-of-the-art knowledge about gastric physiology, which is fundamental to understand pathogenesis and management of type I and type II gNETs.
Jensen, R. T. & Ito, T. in Endotext (eds Feingold, K. et al.) (Endotext, 2023).
Amedei, A. et al. Molecular mimicry between Helicobacter pylori antigens and H+,K+-adenosine triphosphatase in human gastric autoimmunity. J. Exp. Med. 198, 1147–1156 (2003).
Lundell, L., Vieth, M., Gibson, F., Nagy, P. & Kahrilas, P. J. Systematic review: the effects of long‐term proton pump inhibitor use on serum gastrin levels and gastric histology. Aliment. Pharmacol. Ther. 42, 649–663 (2015).
Vanoli, A., Parente, P., Fassan, M., Mastracci, L. & Grillo, F. Gut inflammation and tumorigenesis: every site has a different tale to tell. Intern. Emerg. Med. 18, 2169–2179 (2023).
Arnold, R. et al. Antral gastrin-producing G-cells and somatostatin-producing D-cells in different states of gastric acid secretion. Gut 23, 285–291 (1982).
Annibale, B. et al. Antral gastrin cell hyperfunction and Helicobacter pylori infection. Aliment. Pharmacol. Ther. 10, 607–615 (1996).
Rindi, G. et al. Helicobacter pylori infection in children with antral gastrin cell hyperfunction. J. Pediatr. Gastroenterol. Nutr. 18, 152–158 (1994).
Pashankar, D. S., Israel, D. M., Jevon, G. P. & Buchan, A. M. J. Effect of long-term omeprazole treatment on antral G and D cells in children. J. Pediatr. Gastroenterol. Nutr. 33, 537–542 (2001).
Solcia, E. et al. Gastric carcinoids and related endocrine growths. Digestion 35, 3–22 (1986).
Rindi, G. & Solcia, E. Endocrine hyperplasia and dysplasia in the pathogenesis of gastrointestinal and pancreatic endocrine tumors. Gastroenterol. Clin. North. Am. 36, 851–865 (2007).
Solcia, E. et al. Histopathological classification of nonantral gastric endocrine growths in man. Digestion 41, 185–200 (1988).
Calvete, O. et al. Exome sequencing identifies ATP4A gene as responsible of an atypical familial type I gastric neuroendocrine tumour. Hum. Mol. Genet. 24, 2914–2922 (2015).
Calvete, O. et al. A knockin mouse model for human ATP4a R703C mutation identified in familial gastric neuroendocrine tumors recapitulates the premalignant condition of the human disease and suggests new therapeutic strategies. Dis. Model. Mech. 9, 975–984 (2016).
Langhans, N. et al. Abnormal gastric histology and decreased acid production in cholecystokinin-B/gastrin receptor-deficient mice. Gastroenterology 112, 280–286 (1997).
Benítez, J., Marra, R., Reyes, J. & Calvete, O. A genetic origin for acid–base imbalance triggers the mitochondrial damage that explains the autoimmune response and drives to gastric neuroendocrine tumours. Gastric Cancer 23, 52–63 (2020).
Furlan, D. et al. Different molecular profiles characterize well-differentiated endocrine tumors and poorly differentiated endocrine carcinomas of the gastroenteropancreatic tract. Clin. Cancer Res. 10, 947–957 (2004).
D’Adda, T., Keller, G., Bordi, C. & Höfler, H. Loss of heterozygosity in 11q13-14 regions in gastric neuroendocrine tumors not associated with multiple endocrine neoplasia type 1 syndrome. Lab. Invest. 79, 671–677 (1999).
Toliat, M. R., Berger, W., Ropers, H. H., Neuhaus, P. & Wiedenmann, B. Mutations in the MEN I gene in sporadic neuroendocrine tumours of gastroenteropancreatic system. Lancet 350, 1223 (1997).
Bordi, C. Neuroendocrine pathology of the stomach: the Parma contribution. Endocr. Pathol. 25, 171–180 (2014).
Chatzipanagiotou, O. et al. All you need to know about gastrinoma today | Gastrinoma and Zollinger-Ellison syndrome: a thorough update. J. Neuroendocrinol. 35, e13267 (2023).
Debelenko, L. V. et al. The multiple endocrine neoplasia type I gene locus is involved in the pathogenesis of type II gastric carcinoids. Gastroenterology 113, 773–781 (1997).
Trinh, V. Q., Shi, C. & Ma, C. Gastric neuroendocrine tumours from long‐term proton pump inhibitor users are indolent tumours with good prognosis. Histopathology 77, 865–876 (2020).
Abraham, S. C., Carney, J. A., Ooi, A., Choti, M. A. & Argani, P. Achlorhydria, parietal cell hyperplasia, and multiple gastric carcinoids. Am. J. Surgical Pathol. 29, 969–975 (2005).
Ooi, A. et al. An unusual case of multiple gastric carcinoids associated with diffuse endocrine cell hyperplasia and parietal cell hypertrophy. Endocr. Pathol. 6, 229–237 (1995).
WHO Classification of Tumours Editorial Board. WHO Classification of Tumours: Digestive System Tumours 5th edn, Vol. 1 (WHO, 2019).
Venizelos, A. et al. Germline pathogenic variants in patients with high-grade gastroenteropancreatic neuroendocrine neoplasms. Endocr. Relat. Cancer 30, e230057 (2023).
Taboada, R. et al. Clinicopathological and molecular profile of grade 3 gastroenteropancreatic neuroendocrine neoplasms. J. Neuroendocrinol. 34, e13099 (2022).
Yachida, S. et al. Comprehensive genomic profiling of neuroendocrine carcinomas of the gastrointestinal system. Cancer Discov. 12, 692–711 (2022).
Carabotti, M. et al. Upper gastrointestinal symptoms in autoimmune gastritis: a cross-sectional study. Medicine 96, e5784 (2017).
Lenti, M. V. et al. Cell blood count alterations and patterns of anaemia in autoimmune atrophic gastritis at diagnosis: a multicentre study. J. Clin. Med. 8, 1992 (2019).
Cellini, M. et al. Hashimoto’s thyroiditis and autoimmune gastritis. Front. Endocrinol. 8, 92 (2017).
Kalkan, Ç. & Soykan, I. Polyautoimmunity in autoimmune gastritis. Eur. J. Intern. Med. 31, 79–83 (2016).
Zelissen, P. M., Bast, E. J. & Croughs, R. J. Associated autoimmunity in Addison’s disease. J. Autoimmun. 8, 121–30 (1995).
Dixon, M. F., Genta, R. M., Yardley, J. H. & Correa, P. Classification and grading of gastritis. Am. J. Surg. Pathol. 20, 1161–1181 (1996).
Conti, L. et al. Seronegative autoimmune atrophic gastritis is more common in elderly patients. Dig. Liver Dis. 52, 1310–1314 (2020).
Hershko, C. et al. Variable hematologic presentation of autoimmune gastritis: age-related progression from iron deficiency to cobalamin depletion. Blood 107, 1673–1679 (2006).
Lehy, T., Cadiot, G., Mignon, M., Ruszniewski, P. & Bonfils, S. Influence of multiple endocrine neoplasia type 1 on gastric endocrine cells in patients with the Zollinger-Ellison syndrome. Gut 33, 1275–1279 (1992).
Berna, M. J. et al. A prospective study of gastric carcinoids and enterochromaffin-like cell changes in multiple endocrine neoplasia type 1 and Zollinger-Ellison syndrome: identification of risk factors. J. Clin. Endocrinol. Metab. 93, 1582–1591 (2008).
Roy, P. K. et al. Zollinger-Ellison syndrome: clinical presentation in 261 patients. Medicine 79, 379–411 (2000).
Ito, T., Cadiot, G. & Jensen, R. T. Diagnosis of Zollinger-Ellison syndrome: increasingly difficult. World J. Gastroenterol. 18, 5495–5503 (2012).
Metz, D. C., Cadiot, G., Poitras, P., Ito, T. & Jensen, R. T. Diagnosis of Zollinger-Ellison syndrome in the era of PPIs, faulty gastrin assays, sensitive imaging and limited access to acid secretory testing. Int. J. Endocr. Oncol. 4, 167–185 (2017).
Berna, M. J., Hoffmann, K. M., Serrano, J., Gibril, F. & Jensen, R. T. Serum gastrin in Zollinger-Ellison syndrome: I. Prospective study of fasting serum gastrin in 309 patients from the National Institutes of Health and comparison with 2229 cases from the literature. Medicine 85, 295–330 (2006).
Modlin, I. M. et al. Chromogranin A—biological function and clinical utility in neuro endocrine tumor disease. Ann. Surg. Oncol. 17, 2427–2443 (2010).
Pimentel-Nunes, P. et al. Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) guideline update 2019. Endoscopy 51, 365–388 (2019).
O’Toole, D. et al. AJCC Cancer Staging System, Version nine: Neuroendocrine Tumors of the Stomach (AJCC, 2023).
Deprez, P. H. et al. Endoscopic management of subepithelial lesions including neuroendocrine neoplasms: European Society of Gastrointestinal Endoscopy Guideline. Endoscopy 54, 412–429 (2022).
Borbath, I. et al. ENETS standardized (synoptic) reporting for endoscopy in neuroendocrine tumors. J. Neuroendocrinol. 34, e13105 (2022). This is a reference paper for standardized and high-quality endoscopic assessment of gNETs which is crucial for proper management of these tumours.
Rindi, G. et al. TNM staging of foregut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 449, 395–401 (2006).
Esposito, G. et al. Narrow band imaging characteristics of gastric polypoid lesions: a single-center prospective pilot study. Eur. J. Gastroenterol. Hepatol. 32, 701–705 (2020).
The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest. Endosc. 58, S3–S43 (2003).
Bozkurt, M. F. et al. Guideline for PET/CT imaging of neuroendocrine neoplasms with 68Ga-DOTA-conjugated somatostatin receptor targeting peptides and 18F-DOPA. Eur. J. Nucl. Med. Mol. Imaging 44, 1588–1601 (2017).
Sundin, A. et al. ENETS consensus guidelines for the standards of care in neuroendocrine tumors: radiological, nuclear medicine & hybrid imaging. Neuroendocrinology 105, 212–244 (2017).
Hope, T. A. et al. SNMMI procedure standard/EANM practice guideline for SSTR PET: imaging neuroendocrine tumors. J. Nucl. Med. 64, 204–210 (2023).
Gabriel, M. et al. 68Ga-DOTA-Tyr3-octreotide PET in neuroendocrine tumors: comparison with somatostatin receptor scintigraphy and CT. J. Nucl. Med. 48, 508–518 (2007).
Srirajaskanthan, R. et al. The role of 68Ga-DOTATATE PET in patients with neuroendocrine tumors and negative or equivocal findings on 111In-DTPA-octreotide scintigraphy. J. Nucl. Med. 51, 875–882 (2010).
Skoura, E. et al. The impact of 68Ga-DOTATATE PET/CT imaging on management of patients with neuroendocrine tumors: experience from a national referral center in the United Kingdom. J. Nucl. Med. 57, 34–40 (2016).
Ambrosini, V. et al. 68Ga-DOTA-NOC PET/CT in comparison with CT for the detection of bone metastasis in patients with neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 37, 722–727 (2010).
Putzer, D. et al. Bone metastases in patients with neuroendocrine tumor: 68Ga-DOTA-Tyr3-octreotide PET in comparison to CT and bone scintigraphy. J. Nucl. Med. 50, 1214–1221 (2009).
Rinzivillo, M. et al. Usefulness of 68-gallium PET in type I gastric neuroendocrine neoplasia: a case series. J. Clin. Med. 11, 1641 (2022).
Laird, A. M. & Libutti, S. K. Management of other gastric and duodenal neuroendocrine tumors. Surg. Oncol. Clin. N. Am. 29, 253–266 (2020).
Thomas, D. et al. Long-term follow-up of a large series of patients with type 1 gastric carcinoid tumors: data from a multicenter study. Eur. J. Endocrinol. 168, 185–193 (2013).
Cavallaro, A. et al. The role of 68-Ga-DOTATOC CT-PET in surgical tactic for gastric neuroendocrine tumors treatment: our experience: a case report. Int. J. Surg. 12, S225–S231 (2014).
Auerbach, M. S., Pisegna, J. R., Kim, S. & Yu, R. Three cases of diffuse, intense stomach uptake on DOTATATE PET. Clin. Nucl. Med. 45, 813–816 (2020).
Hope, T. A. et al. Appropriate use criteria for somatostatin receptor PET imaging in neuroendocrine tumors. J. Nucl. Med. 59, 66–74 (2018).
Hicks, R. J. et al. ENETS consensus guidelines for the standards of care in neuroendocrine neoplasms: peptide receptor radionuclide therapy with radiolabelled somatostatin analogues. Neuroendocrinology 105, 295–309 (2017).
Jayasekera, M., Sartin, S. & Bhargava, P. Ga-68 DOTATATE PET/CT in a patient with Zollinger-Ellison syndrome. Radiol. Case Rep. 18, 1046–1048 (2023).
Chan, D. L. et al. Dual [68Ga]DOTATATE and [18F]FDG PET/CT in patients with metastatic gastroenteropancreatic neuroendocrine neoplasms: a multicentre validation of the NETPET score. Br. J. Cancer 128, 549–555 (2023).
Chan, D. L. et al. High metabolic tumour volume on 18-fluorodeoxyglucose positron emission tomography predicts poor survival from neuroendocrine neoplasms. Neuroendocrinology 110, 950–958 (2020).
Binderup, T., Knigge, U., Loft, A., Federspiel, B. & Kjaer, A. 18F-Fluorodeoxyglucose positron emission tomography predicts survival of patients with neuroendocrine tumors. Clin. Cancer Res. 16, 978–985 (2010).
Karfis, I. et al. Prognostic value of a three-scale grading system based on combining molecular imaging with 68Ga-DOTATATE and 18F-FDG PET/CT in patients with metastatic gastroenteropancreatic neuroendocrine neoplasias. Oncotarget 11, 589–599 (2020). This study highlights the clinical importance of functional imaging with both SSTR and 18F-fluorodeoxyglucose PET–CT.
Magi, L. et al. Role of [18F]FDG PET/CT in the management of G1 gastro-entero-pancreatic neuroendocrine tumors. Endocrine 76, 484–490 (2022).
Ambrosini, V. et al. Consensus on molecular imaging and theranostics in neuroendocrine neoplasms. Eur. J. Cancer 146, 56–73 (2021). European consensus on the use of SSTR imaging in NEN, including gNENs.
Chung, C.-S. et al. Clinical features and outcomes of gastric neuroendocrine tumors after endoscopic diagnosis and treatment: a Digestive Endoscopy Society of Tawian (DEST) multicenter study. Medicine 97, e12101 (2018).
Ye, H., Yuan, Y., Chen, P. & Zheng, Q. Risk factors for metastasis and survival of patients with T1 gastric neuroendocrine carcinoma treated with endoscopic therapy versus surgical resection. Surg. Endosc. 36, 6162–6169 (2022).
Merola, E. et al. Type I gastric carcinoids: a prospective study on endoscopic management and recurrence rate. Neuroendocrinology 95, 207–213 (2012).
Uygun, A. et al. Long‐term results of endoscopic resection for type I gastric neuroendocrine tumors. J. Surg. Oncol. 109, 71–74 (2014).
Kim, H. H. et al. The efficacy of endoscopic submucosal dissection of type I gastric carcinoid tumors compared with conventional endoscopic mucosal resection. Gastroenterol. Res. Pract. 2014, 253860 (2014).
Rossi, R. E., Invernizzi, P., Mazzaferro, V. & Massironi, S. Response and relapse rates after treatment with long‐acting somatostatin analogs in multifocal or recurrent type‐1 gastric carcinoids: a systematic review and meta‐analysis. United Eur. Gastroenterol. J. 8, 140–147 (2020).
Campana, D. et al. Clinical management of patients with gastric neuroendocrine neoplasms associated with chronic atrophic gastritis: a retrospective, multicentre study. Endocrine 51, 131–139 (2016). Multicentre retrospective study on the clinical and prognostic characterization of type I gNETs.
Sebastian-Valles, F. et al. Chronic treatment with somatostatin analogues in recurrent type 1 gastric neuroendocrine tumors. Biomedicines 11, 872 (2023).
Boyce, M. et al. Netazepide, a gastrin/cholecystokinin‐2 receptor antagonist, can eradicate gastric neuroendocrine tumours in patients with autoimmune chronic atrophic gastritis. Br. J. Clin. Pharmacol. 83, 466–475 (2017).
Fossmark, R. et al. Treatment of gastric carcinoids type 1 with the gastrin receptor antagonist netazepide (YF476) results in regression of tumours and normalisation of serum chromogranin A. Aliment. Pharmacol. Ther. 36, 1067–1075 (2012).
Thakker, R. V. et al. Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1). J. Clin. Endocrinol. Metab. 97, 2990–3011 (2012).
Delle Fave, G. et al. ENETS consensus guidelines update for gastroduodenal neuroendocrine neoplasms. Neuroendocrinology 103, 119–124 (2016).
Tomassetti, P. et al. Treatment of type II gastric carcinoid tumors with somatostatin analogues. N. Engl. J. Med. 343, 551–554 (2000). Pivotal study showing the effectiveness of somatostatin analogues in the treatment of local unresectable gastrin-dependent type II gNETs.
Exarchou, K. et al. Is local excision sufficient in selected grade 1 or 2 type III gastric neuroendocrine neoplasms? Endocrine 74, 421–429 (2021).
Hanna, A. et al. Gastric neuroendocrine tumors: reappraisal of type in predicting outcome. Ann. Surg. Oncol. 28, 8838–8846 (2021).
White, B. E. et al. Incidence and survival of neuroendocrine neoplasia in England 1995-2018: a retrospective, population-based study. Lancet Reg. Health Eur. 23, 100510 (2022).
Yao, J. C. et al. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet 387, 968–977 (2016).
de Mestier, L. et al. Treatment outcomes of advanced digestive well-differentiated grade 3 NETs. Endocr. Relat. Cancer 28, 549–561 (2021).
Rinke, A. et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J. Clin. Oncol. 27, 4656–4663 (2009).
Pavel, M. et al. Efficacy and safety of high-dose lanreotide autogel in patients with progressive pancreatic or midgut neuroendocrine tumours: CLARINET FORTE phase 2 study results. Eur. J. Cancer 157, 403–414 (2021).
Strosberg, J. et al. Phase 3 trial of 177Lu-Dotatate for midgut neuroendocrine tumors. N. Engl. J. Med. 376, 125–135 (2017).
Xu, J. et al. Surufatinib in advanced extrapancreatic neuroendocrine tumours (SANET-ep): a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 21, 1500–1512 (2020).
Zappi, A. et al. Chemotherapy in well differentiated neuroendocrine tumors (NET) G1, G2, and G3: a narrative review. J. Clin. Med. 12, 717 (2023).
Niederle, B. et al. ENETS consensus guidelines update for neuroendocrine neoplasms of the jejunum and ileum. Neuroendocrinology 103, 125–138 (2016).
Brighi, N. et al. Biliary stone disease in patients receiving somatostatin analogs for neuroendocrine neoplasms. A retrospective observational study. Dig. Liver Dis. 51, 689–694 (2019).
Brighi, N. et al. Biliary stone disease in patients with neuroendocrine tumors treated with somatostatin analogs: a multicenter study. Oncologist 25, 259–265 (2020).
Brabander, T. et al. Long-term efficacy, survival, and safety of [177Lu-DOTA0,Tyr3]octreotate in patients with gastroenteropancreatic and bronchial neuroendocrine tumors. Clin. Cancer Res. 23, 4617–4624 (2017).
Mitjavila, M. et al. Efficacy of [177Lu]Lu-DOTATATE in metastatic neuroendocrine neoplasms of different locations: data from the SEPTRALU study. Eur. J. Nucl. Med. Mol. Imaging 50, 2486–2500 (2023).
Pavel, M. et al. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 31, 844–860 (2020).
Shah, M. H. et al. Neuroendocrine and adrenal tumors, version 2.2021, NCCN clinical practice guidelines in oncology. J. Natl Compr. Cancer Netw. 19, 839–868 (2021).
Ricci, C. et al. Treatment of advanced gastro-entero-pancreatic neuro-endocrine tumors: a systematic review and network meta-analysis of phase III randomized controlled trials. Cancers 13, 358 (2021).
Chan, J. et al. LBA53 Alliance A021602: phase III, double-blinded study of cabozantinib versus placebo for advanced neuroendocrine tumors (NET) after progression on prior therapy (CABINET). Ann. Oncol. 34 (Suppl. 2), S1292 (2023).
Garcia-Carbonero, R. et al. Advances in the treatment of gastroenteropancreatic neuroendocrine carcinomas: are we moving forward? Endocr. Rev. 44, 724–736 (2023).
Sorbye, H. et al. European Neuroendocrine Tumor Society (ENETS) 2023 guidance paper for digestive neuroendocrine carcinoma. J. Neuroendocrinol. 35, e13249 (2023).
Lamberti, G. et al. Targeted genomic profiling and chemotherapy outcomes in grade 3 gastro-entero-pancreatic neuroendocrine tumors (G3 GEP-NET). Diagnostics 13, 1595 (2023).
Dasari, A., Shen, C., Devabhaktuni, A., Nighot, R. & Sorbye, H. Survival according to primary tumor location, stage, and treatment patterns in locoregional gastroenteropancreatic high-grade neuroendocrine carcinomas. Oncologist 27, 299–306 (2022).
Schmitz, R., Mao, R., Moris, D., Strickler, J. H. & Blazer, D. G. Impact of postoperative chemotherapy on the survival of patients with high-grade gastroenteropancreatic neuroendocrine carcinoma. Ann. Surg. Oncol. 28, 114–120 (2021).
Morizane, C. et al. Effectiveness of etoposide and cisplatin vs irinotecan and cisplatin therapy for patients with advanced neuroendocrine carcinoma of the digestive system: the TOPIC-NEC phase 3 randomized clinical trial. JAMA Oncol. 8, 1447–1455 (2022).
McNamara, M. G. et al. NET-02: a randomised, non-comparative, phase II trial of nal-IRI/5-FU or docetaxel as second-line therapy in patients with progressive poorly differentiated extra-pulmonary neuroendocrine carcinoma. EClinicalMedicine 60, 102015 (2023).
Walter, T. et al. Bevacizumab plus FOLFIRI after failure of platinum–etoposide first-line chemotherapy in patients with advanced neuroendocrine carcinoma (PRODIGE 41-BEVANEC): a randomised, multicentre, non-comparative, open-label, phase 2 trial. Lancet Oncol. 24, 297–306 (2023).
Maio, M. et al. Pembrolizumab in microsatellite instability high or mismatch repair deficient cancers: updated analysis from the phase II KEYNOTE-158 study. Ann. Oncol. 33, 929–938 (2022).
Riechelmann, R. P., Taboada, R. G., de Jesus, V. H. F., Iglesia, M. & Trikalinos, N. A. Therapy sequencing in patients with advanced neuroendocrine neoplasms. Am. Soc. Clin. Oncol. Educ. Book. 43, e389278 (2023). ASCO educational book about the challenging management of advanced metastatic NEN, including gNENs.
Subbiah, V. et al. Pan-cancer efficacy of pralsetinib in patients with RET fusion-positive solid tumors from the phase 1/2 ARROW trial. Nat. Med. 28, 1640–1645 (2022).
Sigal, D. S. et al. Comprehensive genomic profiling identifies novel NTRK fusions in neuroendocrine tumors. Oncotarget 9, 35809–35812 (2018).
Doebele, R. C. et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1–2 trials. Lancet Oncol. 21, 271–282 (2020).
Hong, D. S. et al. Larotrectinib in patients with TRK fusion-positive solid tumours: a pooled analysis of three phase 1/2 clinical trials. Lancet Oncol. 21, 531–540 (2020).
Idrees, K. et al. Frequent BRAF mutations suggest a novel oncogenic driver in colonic neuroendocrine carcinoma. J. Surg. Oncol. 117, 284–289 (2018).
Singh, S. et al. Patient-reported burden of a neuroendocrine tumor (NET) diagnosis: results from the first global survey of patients with NETs. J. Glob. Oncol. 3, 43–53 (2017).
Gosain, R. et al. Health-related quality of life (HRQoL) in neuroendocrine tumors: a systematic review. Cancers 14, 1428 (2022).
Yadegarfar, G. et al. Validation of the EORTC QLQ-GINET21 questionnaire for assessing quality of life of patients with gastrointestinal neuroendocrine tumours. Br. J. Cancer 108, 301–310 (2013).
Strosberg, J. et al. Health-related quality of life in patients with progressive midgut neuroendocrine tumors treated with 177Lu-Dotatate in the phase III NETTER-1 trial. J. Clin. Oncol. 36, 2578–2584 (2018).
Strosberg, J. R. et al. Symptom diaries of patients with midgut neuroendocrine tumors treated with 177Lu-DOTATATE. J. Nucl. Med. 62, 1712–1718 (2021).
Pavel, M. E. et al. Health-related quality of life for everolimus versus placebo in patients with advanced, non-functional, well-differentiated gastrointestinal or lung neuroendocrine tumours (RADIANT-4): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 18, 1411–1422 (2017).
Ramage, J. K. et al. Observational study to assess quality of life in patients with pancreatic neuroendocrine tumors receiving treatment with everolimus: the OBLIQUE study (UK phase IV trial). Neuroendocrinology 108, 317–327 (2019).
Peipert, B. J., Goswami, S., Yount, S. E. & Sturgeon, C. Health-related quality of life in MEN1 patients compared with other chronic conditions and the United States general population. Surgery 163, 205–211 (2018).
Author information
Authors and Affiliations
Contributions
Introduction (G.L., G.R. and D.C.); Epidemiology (F.P.); Mechanisms/pathophysiology (G.R.), Diagnosis, screening and prevention (G.L., M.P., D.O’T. and V.A.), Management (G.L., F.P., M.F., R.G.-C. and D.C.), Quality of life (R.P.R.), Outlook (G.L., G.R. and D.C.); overview of Primer (D.C. and G.R.). G.L. and F.P. contributed equally and are co-first authors; D.C. and G.R. contributed equally and are co-last authors.
Corresponding author
Ethics declarations
Competing interests
M.P. reports honoraria for presentations from Novartis, IPSEN, AAA, Boehringer Ingelheim, MSD, Recordati, Lilly and Sanofi; honoraria for advisory board or consultancy from AAA, IPSEN, Riemser and Hutchmed; fees to institution clinical trials from Novartis, ITM, AAA and IPSEN. V.A. reports honoraria for presentations from ESMIT, EANM/ESMO, AAA, Cineca and Elma Academy outside the submitted work. G.R. reports personal fees from AAA (invited speaker, speaker bureau), Bracco Imaging (consultancy) and being a reviewer for a grant application for GIMBE Foundation (Gruppo Italiano Medicina Fondata sull’Evidenza). D.C. reports honoraria for presentations from AAA, Ipsen, Novartis and Esteve. All other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Disease Primers thanks Mark Pritchard, Christos Toumpanakis, Helge Waldum and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lamberti, G., Panzuto, F., Pavel, M. et al. Gastric neuroendocrine neoplasms. Nat Rev Dis Primers 10, 25 (2024). https://doi.org/10.1038/s41572-024-00508-y
Accepted:
Published:
DOI: https://doi.org/10.1038/s41572-024-00508-y