Abstract
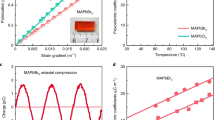
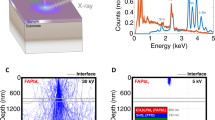
In the same way as electron transport is crucial for information technology, ion transport is a key phenomenon in the context of energy research. To be able to tune ion conduction by light would open up opportunities for a wide realm of new applications, but it has been challenging to provide clear evidence for such an effect. Here we show through various techniques, such as transference-number measurements, permeation studies, stoichiometric variations, Hall effect experiments and the use of blocking electrodes, that light excitation enhances by several orders of magnitude the ionic conductivity of methylammonium lead iodide, the archetypal metal halide photovoltaic material. We provide a rationale for this unexpected phenomenon and show that it straightforwardly leads to a hitherto unconsidered photodecomposition path of the perovskite.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Merkle, R., de Souza, R. A. & Maier, J. Optically tuning the rate of stoichiometry changes: surface-controlled oxygen incorporation into oxides under UV irradiation. Angew. Chem. Int. Ed. 40, 2126–2129 (2001).
Walch, G. et al. A solid oxide photoelectrochemical cell with UV light-driven oxygen storage in mixed conducting electrodes. J. Mater. Chem. A 5, 1637–1649 (2017).
Boyden, E. S., Zhang, F., Bamberg, E., Nagel, G. & Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 8, 1263–1268 (2005).
Brenner, T. M., Egger, D. A., Kronik, L., Hodes, G. & Cahen, D. Hybrid organic–inorganic perovskites: low-cost semiconductors with intriguing charge-transport properties. Nat. Rev. Mater. 1, 15007 (2016).
Dequilettes, D. W. et al. Photo-induced halide redistribution in organic–inorganic perovskite films. Nat. Commun. 7, 11683 (2016).
Eames, C. et al. Ionic transport in hybrid lead iodide perovskite solar cells. Nat. Commun. 6, 7497 (2015).
Hoke, E. T. et al. Reversible photo-induced trap formation in mixed-halide hybrid perovskites for photovoltaics. Chem. Sci. 6, 613–617 (2015).
Ummadisingu, A. et al. The effect of illumination on the formation of metal halide perovskite films. Nature 545, 208–212 (2017).
Leijtens, T. et al. Mapping electric field-induced switchable poling and structural degradation in hybrid lead halide perovskite thin films. Adv. Energy Mater. 5, 1500962 (2015).
Meloni, S. et al. Ionic polarization-induced current–voltage hysteresis in CH3NH3PbX3 perovskite solar cells. Nat. Commun. 7, 10334 (2016).
Yuan, Y. & Huang, J. Ion migration in organometal trihalide perovskite and its impact on photovoltaic efficiency and stability. Acc. Chem. Res. 49, 286–293 (2016).
Green, M. A., Ho-Baillie, A. & Snaith, H. J. The emergence of perovskite solar cells. Nat. Photon. 8, 506–514 (2014).
Jung, H. S. & Park, N.-G. Perovskite solar cells: from materials to devices. Small 11, 10–25 (2015).
Kojima, A., Teshima, K., Shirai, Y. & Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 131, 6050–6051 (2009).
Yang, W. S. et al. Iodide management in formamidinium–lead–halide-based perovskite layers for efficient solar cells. Science 356, 1376–1379 (2017).
Yang, T. Y., Gregori, G., Pellet, N., Gratzel, M. & Maier, J. The significance of ion conduction in a hybrid organic–inorganic lead-iodide-based perovskite photosensitizer. Angew. Chem. Int. Ed. 54, 7905–7910 (2015).
Senocrate, A. et al. The nature of ion conduction in methylammonium lead iodide: a multimethod approach. Angew. Chem. Int. Ed. 56, 7755–7759 (2017).
Xing, J. et al. Ultrafast ion migration in hybrid perovskite polycrystalline thin films under light and suppression in single crystals. Phys. Chem. Chem. Phys. 18, 30484–30490 (2016).
Zhao, Y.-C. et al. Quantification of light-enhanced ionic transport in lead iodide perovskite thin films and its solar cell applications. Light Sci. Appl. 6, e16243 (2017).
Sabeth, F., Iimori, T. & Ohta, N. Gigantic photoresponse and reversible photoswitching in ionic conductivity of polycrystalline β-AgI. J. Phys. Chem. C 116, 9209–9213 (2012).
Mosconi, E., Meggiolaro, D., Snaith, H. J., Stranks, S. D. & de Angelis, F. Light-induced annihilation of Frenkel defects in organo–lead halide perovskites. Energy Environ. Sci. 9, 3180–3187 (2016).
Maier, J. Physical Chemistry of Ionic Materials: Ions and Electrons in Solids (Wiley, Chichester, 2004).
Maier, J. in Modern Aspects of Electrochemistry No. 41 (eds C. Vayenas, R. E. White & M. E. Gamboa-Aldeco) 1–138 (Springer, New York, NY, 2007).
Zohar, A. et al. What Is the mechanism of MAPbI3 p-doping by I2? Insights from optoelectronic properties. ACS Energy Lett. 2, 2408–2414 (2017).
Wagner, C. Galvanic cells with solid electrolytes involving ionic and electronic conduction. In Int. Committee of Electrochemical Thermodynamics and Kinetics, Proc. of the Seventh Meeting at Lindau 1955 361–377 (Butterworth Scientific, London 1957).
Rickert, H. Electrochemistry of Solids (Springer, Berlin, Heidelberg, 1982).
Wang, S., Jiang, Y., Juarez-Perez, E. J., Ono, L. K. & Qi, Y. Accelerated degradation of methylammonium lead iodide perovskites induced by exposure to iodine vapour. Nat. Energy 2, 16195 (2016).
Mazumdar, N., Chikindas, M. L. & Uhrich, K. Slow release polymer–iodine tablets for disinfection of untreated surface water. J. Appl. Polym. Sci. 117, 329–334 (2010).
Walsh, A., Scanlon, D. O., Chen, S., Gong, X. G. & Wei, S.-H. Self-regulation mechanism for charged point defects in hybrid halide perovskites. Angew. Chem. Int. Ed. 54, 1791–1794 (2015).
Tang, X. F. et al. Photoinduced degradation of methylammonium lead triiodide perovskite semiconductors. J. Mater. Chem. A 4, 15896–15903 (2016).
Meloni, S., Palermo, G., Ashari-Astani, N., Gratzel, M. & Rothlisberger, U. Valence and conduction band tuning in halide perovskites for solar cell applications. J. Mater. Chem. A 4, 15997–16002 (2016).
Yin, W. J., Shi, T. T. & Yan, Y. F. Unusual defect physics in CH3NH3PbI3 perovskite solar cell absorber. Appl. Phys. Lett. 104, 063903 (2014).
Popov, A. I., Kotomin, E. A. & Maier, J. Analysis of self-trapped hole mobility in alkali halides and metal halides. Solid State Ionics 302, 3–6 (2017).
Loftager, S., García-Fernández, P., Aramburu, J. A., Moreno, M. & Garcia-Lastra, J. M. Stability and polaronic motion of self-trapped holes in silver halides: insight through DFT+ U calculations. J. Phys. Chem. C 120, 8509–8524 (2016).
Brudevoll, T., Kotomin, E. A. & Christensen, N. E. Interstitial-oxygen–atom diffusion in MgO. Phys. Rev. B 53, 7731–7735 (1996).
Murray, R. B. & Keller, F. J. Vk Centers and recombination luminescence in rubidium iodide and sodium iodide. Phys. Rev. 153, 993–999 (1967).
Wilson, D. J., Sokol, A. A., French, S. A. & Catlow, C. R. A. Defect structures in the silver halides. Phys. Rev. B 77, 064115 (2008).
Du, M. H. Efficient carrier transport in halide perovskites: theoretical perspectives. J. Mater. Chem. A 2, 9091–9098 (2014).
Whalley, L. D., Crespo-Otero, R. & Walsh, A. H-center and V-center defects in hybrid halide perovskites. ACS Energy Lett. 2, 2713–2714 (2017).
Li, W., Liu, J., Bai, F.-Q., Zhang, H.-X. & Prezhdo, O. V. Hole trapping by iodine interstitial defects decreases free carrier losses in perovskite solar cells: a time-domain ab initio study. ACS Energy Lett. 2, 1270–1278 (2017).
Maier, J. Mass transport in the presence of internal defect reactions—concept of conservative ensembles: I, chemical diffusion in pure compounds. J. Am. Ceram. Soc. 76, 1212–1217 (1993).
Im, J.-H., Lee, C.-R., Lee, J.-W., Park, S.-W. & Park, N.-G. 6.5% efficient perovskite quantum-dot-sensitized solar cell. Nanoscale 3, 4088–4093 (2011).
Jeon, N. J. et al. Solvent engineering for high-performance inorganic–organic hybrid perovskite solar cells. Nat. Mater. 13, 897 (2014).
Gillespie, L. J. & Fraser, L. H. D. The normal vapor pressure of crystalline iodine. J. Am. Chem. Soc. 58, 2260–2263 (1936).
Acknowledgements
The authors are grateful to H. Hoier and G. Maier for XRD measurements, J. Na for his help with the Hall-effect experiments and A. Güth (Nanostructuring Lab in the Max Planck Institute for Solid State Research) for his help with electrode deposition. They are indebted to K. Müller from the Scientific Facility for Interface Analysis (headed by U. Starke) for XPS measurements. The authors also thank E. Kotomin, R. Evarestov, J. Fleig and, in particular, R. Merkle for many helpful discussions. This work was performed within the framework of the Max Planck-EPFL Center for Molecular Nano-science and Technology.
Author information
Authors and Affiliations
Contributions
G.Y.K. was responsible for the sample preparation and characterization. G.Y.K., A.S., T.-Y.Y., G.G. and J.M. designed the experiments. J.M. supervised the work. G.Y.K., A.S., M.G. and J.M. discussed the results and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary text (9 sections), Figures S1–S10, 17 references
Rights and permissions
About this article
Cite this article
Kim, G.Y., Senocrate, A., Yang, TY. et al. Large tunable photoeffect on ion conduction in halide perovskites and implications for photodecomposition. Nature Mater 17, 445–449 (2018). https://doi.org/10.1038/s41563-018-0038-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-018-0038-0
This article is cited by
-
Mobile iodides capture for highly photolysis- and reverse-bias-stable perovskite solar cells
Nature Materials (2024)
-
High-performance photodetectors based on low-defect CsPb1-xZnxBr3 quantum dots
Journal of Materials Science: Materials in Electronics (2024)
-
Ion-induced field screening as a dominant factor in perovskite solar cell operational stability
Nature Energy (2024)
-
Mapping the pathways of photo-induced ion migration in organic-inorganic hybrid halide perovskites
Nature Communications (2023)
-
Long-term operating stability in perovskite photovoltaics
Nature Reviews Materials (2023)