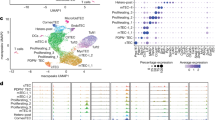
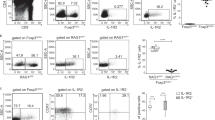
Abstract
Newborn animals require tightly regulated local and systemic immune environments to govern the development and maturation of multiple organs/tissues even though the immune system itself is far from mature during the neonatal period. Regulatory T cells (Tregs) are essential for maintaining immune tolerance/homeostasis and modulating inflammatory responses. The features of Tregs in the neonatal liver under steady-state conditions are not well understood. The present study aimed to investigate the phenotype, functions, and significance of neonatal Tregs in the liver. We found a wave of thymus-derived Treg influx into the liver during 1–2 weeks of age. Depletion of these Tregs between days 7 and 11 after birth rapidly resulted in Th1-type liver inflammation and metabolic disorder. More Tregs in the neonatal liver than in the spleen underwent MHC II-dependent activation and proliferation, and the liver Tregs acquired stronger suppressive functions. The transcriptomic profile of these neonatal liver Tregs showed elevated expression of PPARγ and T-bet and features of Tregs that utilize lipid metabolic machinery and are capable of regulating Th1 responses. The accumulation of Tregs with unique features in the neonatal liver is critical to ensure self-tolerance and liver maturation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Grijalva, J. & Vakili, K. Neonatal liver physiology. Semin Pedia. Surg. 22, 185–189 (2013).
Nakagaki B. N., et al. Immune and metabolic shifts during neonatal development reprogram liver identity and function. J. Hepatol. 69, 1294–1307 (2018).
van Elburg, R. M., Fetter, W. P., Bunkers, C. M. & Heymans, H. S. Intestinal permeability in relation to birth weight and gestational and postnatal age. Arch. Dis. Child Fetal Neonatal Ed. 88, F52–55 (2003).
Weaver, L. T., Laker, M. F., Nelson, R. & Lucas, A. Milk feeding and changes in intestinal permeability and morphology in the newborn. J. Pedia. Gastroenterol. Nutr. 6, 351–358 (1987).
Prandota, J. Possible pathomechanisms of sudden infant death syndrome: key role of chronic hypoxia, infection/inflammation states, cytokine irregularities, and metabolic trauma in genetically predisposed infants. Am. J. Ther. 11, 517–546 (2004).
Godfrey, V. L., Wilkinson, J. E. & Russell, L. B. X-linked lymphoreticular disease in the scurfy (sf) mutant mouse. Am. J. Pathol. 138, 1379–1387 (1991).
Fontenot, J. D., Gavin, M. A. & Rudensky, A. Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 4, 330–336 (2003).
Huter, E. N. et al. TGF-beta-induced Foxp3+ regulatory T cells rescue scurfy mice. Eur. J. Immunol. 38, 1814–1821 (2008).
Fontenot, J. D. et al. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity 22, 329–341 (2005).
Yang, S., Fujikado, N., Kolodin, D., Benoist, C. & Mathis, D. Immune tolerance. Regulatory T cells generated early in life play a distinct role in maintaining self-tolerance. Science 348, 589–594 (2015).
Lathrop, S. K. et al. Peripheral education of the immune system by colonic commensal microbiota. Nature 478, 250–254 (2011).
Hayakawa, S., Ohno, N., Okada, S. & Kobayashi, M. Significant augmentation of regulatory T cell numbers occurs during the early neonatal period. Clin. Exp. Immunol. 190, 268–279 (2017).
Maria, A., English, K. A. & Gorham, J. D. Appropriate development of the liver Treg compartment is modulated by the microbiota and requires TGF-beta and MyD88. J. Immunol. Res. 2014, 279736 (2014).
Tucker, R. M., Feldman, A. G., Fenner, E. K. & Mack, C. L. Regulatory T cells inhibit Th1 cell-mediated bile duct injury in murine biliary atresia. J. Hepatol. 59, 790–796 (2013).
Lages, C. S., Simmons, J., Chougnet, C. A. & Miethke, A. G. Regulatory T cells control the CD8 adaptive immune response at the time of ductal obstruction in experimental biliary atresia. Hepatology 56, 219–227 (2012).
Wawman, R. E., Bartlett, H. & Oo, Y. H. Regulatory T cell metabolism in the hepatic microenvironment. Front Immunol. 8, 1889 (2017).
Chen, Y. Y. et al. Human intrahepatic regulatory T cells are functional, require IL-2 from effector cells for survival, and are susceptible to Fas ligand-mediated apoptosis. Hepatology 64, 138–150 (2016).
Thornton, A. M. et al. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J. Immunol. 184, 3433–3441 (2010).
Weiss, J. M. et al. Neuropilin 1 is expressed on thymus-derived natural regulatory T cells, but not mucosa-generated induced Foxp3+ T reg cells. J. Exp. Med. 209, 1723–1742 (2012). S1721.
Yadav, M. et al. Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. J. Exp. Med. 209, 1713–1722 (2012). S1711–1719.
Tuncel J., Benoist C., Mathis D. T cell anergy in perinatal mice is promoted by T reg cells and prevented by IL-33. J. Exp. Med. 2019, Apr 15. pii: jem.20182002. https://doi.org/10.1084/jem.20182002.
Attias M., Al-Aubodah T., Piccirillo C. A. Mechanisms of human FoxP3(+) Treg cell development and function in health and disease. Clin. Exp. Immunol. 2019, Mar 12. https://doi.org/10.1111/cei.13290.
Levine, A. G., Arvey, A., Jin, W. & Rudensky, A. Y. Continuous requirement for the TCR in regulatory T cell function. Nat. Immunol. 15, 1070–1078 (2014).
Miao, T. et al. Egr2 and 3 control adaptive immune responses by temporally uncoupling expansion from T cell differentiation. J. Exp. Med 214, 1787–1808 (2017).
Levine, A. G. et al. Stability and function of regulatory T cells expressing the transcription factor T-bet. Nature 546, 421–425 (2017).
Littringer, K. et al. Common features of regulatory T cell specialization during Th1 responses. Front Immunol. 9, 1344 (2018).
Cipolletta, D., Cohen, P., Spiegelman, B. M., Benoist, C. & Mathis, D. Appearance and disappearance of the mRNA signature characteristic of Treg cells in visceral adipose tissue: age, diet, and PPARgamma effects. Proc. Natl Acad. Sci. USA 112, 482–487 (2015).
Li, C. et al. TCR transgenic mice reveal stepwise, multi-site acquisition of the distinctive fat-treg phenotype. Cell 174, 285–299 (2018). e212.
Leventhal, D. S. et al. Dendritic cells coordinate the development and homeostasis of organ-specific regulatory T cells. Immunity 44, 847–859 (2016).
Vahl, J. C. et al. Continuous T cell receptor signals maintain a functional regulatory T cell pool. Immunity 41, 722–736 (2014).
Malchow, S. et al. Aire enforces immune tolerance by directing autoreactive T cells into the regulatory T cell lineage. Immunity 44, 1102–1113 (2016).
Metzger, T. C. & Anderson, M. S. Control of central and peripheral tolerance by Aire. Immunol. Rev. 241, 89–103 (2011).
Gardner, J. M. et al. Deletional tolerance mediated by extrathymic Aire-expressing cells. Science 321, 843–847 (2008).
Liston, A., Lesage, S., Wilson, J., Peltonen, L. & Goodnow, C. C. Aire regulates negative selection of organ-specific T cells. Nat. Immunol. 4, 350–354 (2003).
Lindmark, E. et al. AIRE expressing marginal zone dendritic cells balances adaptive immunity and T-follicular helper cell recruitment. J. Autoimmun. 42, 62–70 (2013).
Hirayama, K. et al. Development of intestinal flora of human-flora-associated (HFA) mice in the intestine of their offspring. Exp. Anim. 44, 219–222 (1995).
Schrage, A. et al. Enhanced T cell transmigration across the murine liver sinusoidal endothelium is mediated by transcytosis and surface presentation of chemokines. Hepatology 48, 1262–1272 (2008).
Scharschmidt, T. C. et al. A wave of regulatory T cells into neonatal skin mediates tolerance to commensal microbes. Immunity 43, 1011–1021 (2015).
Scharschmidt, T. C. et al. Commensal microbes and hair follicle morphogenesis coordinately drive treg migration into neonatal skin. Cell Host Microbe 21, 467–477 (2017). e465.
Hashimoto, K. & Ogawa, Y. Epigenetic switching and neonatal nutritional environment. Adv. Exp. Med. Biol. 1012, 19–25 (2018).
Yang, S. L. et al. Associations between inflammatory cytokines and organ damage in pediatric patients with hemophagocytic lymphohistiocytosis. Cytokine 85, 14–17 (2016).
Mack, C. L. et al. Biliary atresia is associated with CD4+ Th1 cell-mediated portal tract inflammation. Pedia. Res. 56, 79–87 (2004).
Lakshminarayanan, B. & Davenport, M. Biliary atresia: a comprehensive review. J. Autoimmun. 73, 1–9 (2016).
Koch, M. A. et al. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat. Immunol. 10, 595–602 (2009).
Nosko, A. et al. T-bet enhances regulatory T cell fitness and directs control of Th1 responses in crescentic GN. J. Am. Soc. Nephrol. 28, 185–196 (2017).
Xiong, Y., Ahmad, S., Iwami, D., Brinkman, C. C. & Bromberg, J. S. T-bet regulates natural regulatory T cell afferent lymphatic migration and suppressive function. J. Immunol. 196, 2526–2540 (2016).
Kumar, S. K. & Bhat, B. V. Distinct mechanisms of the newborn innate immunity. Immunol. Lett. 173, 42–54 (2016).
Decaux, J. F., Ferre, P., Robin, D., Robin, P. & Girard, J. Decreased hepatic fatty acid oxidation at weaning in the rat is not linked to a variation of malonyl-CoA concentration. J. Biol. Chem. 263, 3284–3289 (1988).
Feuerer, M. et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat. Med. 15, 930–939 (2009).
Vasanthakumar, A. et al. The transcriptional regulators IRF4, BATF and IL-33 orchestrate development and maintenance of adipose tissue-resident regulatory T cells. Nat. Immunol. 16, 276–285 (2015).
Smigiel, K. S. et al. CCR7 provides localized access to IL-2 and defines homeostatically distinct regulatory T cell subsets. J. Exp. Med. 211, 121–136 (2014).
Wing, J. B. et al. A distinct subpopulation of CD25(-) T-follicular regulatory cells localizes in the germinal centers. Proc. Natl Acad. Sci. USA 114, E6400–E6409 (2017).
Ferreira, R. C. et al. Cells with Treg-specific FOXP3 demethylation but low CD25 are prevalent in autoimmunity. J. Autoimmun. 84, 75–86 (2017).
Toomer K. H., Malek T. R. Cytokine signaling in the development and homeostasis of regulatory T cells. Cold Spring Harb. Perspect. Biol. 2018; 10. pii: a028597. https://doi.org/10.1101/cshperspect.a028597.
Raynor, J. et al. IL-6 and ICOS antagonize bim and promote regulatory T cell accrual with age. J. Immunol. 195, 944–952 (2015).
Acknowledgements
We wish to thank Professors Yu Zhang, Jun Zhang, Wenling Han, Ying Wang, Chao Zhong, Xiaoyan Qiu, and Dan Lv (Peking University, China) for critical comments, helpful discussions, and critical reagents. This work was supported by grants from the National Key Research and Development Program of China (2017YFA0104500), the National Natural Science Foundation of China (81471525, 31671244, and 31872734, Q.G.; 81601975, K.Z.), the Foundation for Innovative Research Groups of the National Natural Science Foundation of China (81621001), and the Non-Profit Central Research Institute Fund of Chinese Academy of Medical Sciences (2018PT31039).
Author information
Authors and Affiliations
Contributions
Q.G., M.L., and K.Z. designed the research, analyzed the data, and wrote the paper. M.L., W.Z., L.J., and Y.W. performed the research. W.W., K.W., J.H., R.J., X.S., X.X., Y.C., J.G., X.H., Y.S., and W.F. contributed reagents and technical support. H.W. helped with flow cytometry. X.Z. and K.Z. edited the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Li, M., Zhao, W., Wang, Y. et al. A wave of Foxp3+ regulatory T cell accumulation in the neonatal liver plays unique roles in maintaining self-tolerance. Cell Mol Immunol 17, 507–518 (2020). https://doi.org/10.1038/s41423-019-0246-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41423-019-0246-9
Keywords
This article is cited by
-
Gut–liver axis: barriers and functional circuits
Nature Reviews Gastroenterology & Hepatology (2023)
-
Bcl10 is required for the development and suppressive function of Foxp3+ regulatory T cells
Cellular & Molecular Immunology (2021)
-
Functional heterogeneity of CD4+ T cells in liver inflammation
Seminars in Immunopathology (2021)