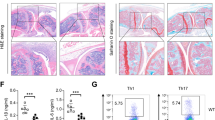
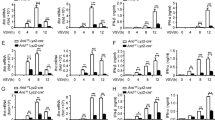
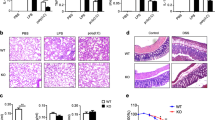
Abstract
Epigenetic modification, including histone modification, precisely controls target gene expression. The posttranscriptional regulation of the innate signaling-triggered production of inflammatory cytokines and type I interferons has been fully elucidated, whereas the roles of histone modification alteration and epigenetic modifiers in regulating inflammatory responses need to be further explored. Di/tri-methylation modifications of histone 3 lysine 79 (H3K79me2/3) have been shown to be associated with gene transcriptional activation. Disruptor of telomeric silencing-1-like (Dot1l) is the only known exclusive H3K79 methyltransferase and regulates the proliferation and differentiation of tumor cells. However, the roles of Dot1l and Dot1l-mediated H3K79 methylation in innate immunity and inflammatory responses remain unclear. Here, we found that H3K79me2/3 modification levels at the Il6 and Ifnb1 promoters, as well as H3K79me2 modification at the Tnfα promoter, were increased in macrophages activated by Toll-like receptor (TLR) ligands or virus infection. The innate signals upregulated Dot1l expression in macrophages and THP1 cells. Dot1l silencing or a Dot1l inhibitor preferentially suppressed the production of IL-6 and interferon (IFN)-β but not of TNF-α in macrophages and THP1 cells triggered by TLR ligands or virus infection. Dot1l was recruited to the proximal promoter of the Il6 and Ifnb1 but not Tnfα gene and then mediated H3K79me2/3 modification at the Il6 and Ifnb1 promoters, consequently facilitating the transcription and expression of Il6 and Ifnb1. Thus, Dot1l-mediated selective H3K79me2/3 modifications at the Il6 and Ifnb1 promoters are required for the full activation of innate immune responses. This finding adds new insights into the epigenetic regulation of inflammatory responses and pathogenesis of autoimmune diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Kieser, K. J. & Kagan, J. C. Multi-receptor detection of individual bacterial products by the innate immune system. Nat. Rev. Immunol. 17, 376–390 (2017).
Lee, M. S. & Kim, Y. J. Signaling pathways downstream of pattern-recognition receptors and their cross talk. Annu. Rev. Biochem. 76, 447–480 (2007).
Goubau, D., Deddouche, S. & Reis e Sousa, C. Cytosolic sensing of viruses. Immunity 38, 855–869 (2013).
Takeuchi, O. & Akira, S. Pattern-recognition receptors and inflammation. Cell 140, 805–820 (2010).
Cao, X. Self-regulation and cross-regulation of pattern-recognition receptor signalling in health and disease. Nat. Rev. Immunol. 16, 35–50 (2016).
Liu, X. et al. MicroRNA in vivo precipitation identifies miR-151-3p as a computational unpredictable miRNA to target Stat3 and inhibits innate IL-6 production. Cell Mol Immunol. 15, 99–110 (2018).
Chiang, C. & Gack, M. U. Post-translational control of intracellular pathogen sensing pathways. Trends Immunol. 38, 39–52 (2017).
Zhang H., Han C., Li T., Li N., Cao X. The methyltransferase PRMT6 attenuates antiviral innate immunity by blocking TBK1-IRF3 signaling. Cell Mol Immunol. 2018; in press.
Zhong, Q. et al. IgG immunocomplexes sensitize human monocytes for inflammatory hyperactivity via transcriptomic and epigenetic reprogramming in rheumatoid arthritis. J. Immunol. 200, 3913–3925 (2018).
Sayed, N. et al. Retinoic acid inducible gene 1 protein (RIG1)-like receptor pathway is required for efficient nuclear reprogramming. Stem Cells 35, 1197–1207 (2017).
Grazioli, E. et al. Physical activity in the prevention of human diseases: role of epigenetic modifications. BMC Genomics 18, 802 (2017).
Greer, E. L. & Shi, Y. Histone methylation: a dynamic mark in health, disease and inheritance. Nat. Rev. Genet. 13, 343–357 (2012).
Zhang, Q. et al. Tet2 is required to resolve inflammation by recruiting Hdac2 to specifically repress IL-6. Nature 525, 389–393 (2015).
Black, J. C., Van Rechem, C. & Whetstine, J. R. Histone lysine methylation dynamics: establishment, regulation, and biological impact. Mol. Cell 48, 491–507 (2012).
Xia, M. et al. Histone methyltransferase Ash1l suppresses interleukin-6 production and inflammatory autoimmune diseases by inducing the ubiquitin-editing enzyme A20. Immunity 39, 470–481 (2013).
Liu, Y. et al. Histone lysine methyltransferase Ezh1 promotes TLR-triggered inflammatory cytokine production by suppressing Tollip. J. Immunol. 194, 2838–2846 (2015).
Li, X. et al. Demethylase Kdm6a epigenetically promotes IL-6 and IFN-β production in macrophages. J. Autoimmun. 80, 85–94 (2017).
Kruidenier, L. et al. A selective jumonji H3K27 demethylase inhibitor modulates the proinflammatory macrophage response. Nature 488, 404–408 (2012).
Schliehe, C. et al. The methyltransferase Setdb2 mediates virus-induced susceptibility to bacterial superinfection. Nat. Immunol. 16, 67–74 (2015).
McLean, C. M., Karemaker, I. D. & van Leeuwen, F. The emerging roles of Dot1l in leukemia and normal development. Leukemia 28, 2131–2138 (2014).
Farooq, Z., Banday, S., Pandita, T. K. & Altaf, M. The many faces of histone H3K79 methylation. Mutat. Res. Rev. Mutat. Res 768, 46–52 (2016).
Bernt, K. M. et al. MLL-rearranged leukemia is dependent on aberrant H3K79 methylation by DOT1L. Cancer Cell. 20, 66–78 (2011).
Wong, M. et al. The histone methyltransferase DOT1L promotes neuroblastoma by regulating gene transcription. Cancer Res. 77, 2522–2533 (2017).
Gao, Y. & Ge, W. The histone methyltransferase DOT1L inhibits osteoclastogenesis and protects against osteoporosis. Cell Death Dis. 9, 33 (2018).
Castaño Betancourt, M. C. et al. Genome-wide association and functional studies identify the DOT1L gene to be involved in cartilage thickness and hip osteoarthritis. Proc. Natl Acad. Sci. USA 109, 8218–8223 (2012).
Shen, Q. et al. Tet2 promotes pathogen infection-induced myelopoiesis through mRNA oxidation. Nature 554, 123–127 (2018).
Schübeler, D. et al. The histone modification pattern of active genes revealed through genome-wide chromatin analysis of a higher eukaryote. Genes Dev. 18, 1263–1271 (2004).
Pokholok, D. K. et al. Genome-wide map of nucleosome acetylation and methylation in yeast. Cell 122, 517–527 (2005).
Vakoc, C. R., Sachdeva, M. M., Wang, H. & Blobel, G. A. Profile of histone lysine methylation across transcribed mammalian chromatin. Mol. Cell Biol. 26, 9185–9195 (2006).
Marson, A. et al. Connecting microRNA genes to the core transcriptional regulatory circuitry of embryonic stem cells. Cell 134, 521–533 (2008).
Cho, M. H. et al. DOT1L cooperates with the c-Myc-p300 complex to epigenetically derepress CDH1 transcription factors in breast cancer progression. Nat. Commun. 6, 7821 (2015).
Feinberg, A. P. Phenotypic plasticity and the epigenetics of human disease. Nature 447, 433–440 (2007).
Nishina, N. et al. Baseline levels of soluble interleukin-6 receptor predict clinical remission in patients with rheumatoid arthritis treated with tocilizumab: implications for molecular targeted therapy. Ann. Rheum. Dis. 73, 945–947 (2014).
Fiedler SE, et al. Analysis of IL-6, IL-1β and TNF-α production in monocytes isolated from multiple sclerosis patients treated with disease modifying drugs. J. Syst. Integr. Neurosci. 2017; 3. https://doi.org/10.15761/JSIN.1000166.
Hagberg, N. & Rönnblom, L. Systemic lupus erythematosus-a disease with A dysregulated type I interferon system. Scand. J. Immunol. 82, 199–207 (2015).
Theofilopoulos, A. N., Baccala, R., Beutler, B. & Kono, D. H. Type I interferons (alpha/beta) in immunity and autoimmunity. Annu. Rev. Immunol. 23, 307–336 (2005).
Aslani, S. et al. Epigenetic alterations underlying autoimmune diseases. Autoimmunity 49, 69–83 (2016).
Yarilina, A., Park-Min, K. H., Antoniv, T., Hu, X. & Ivashkiv, L. B. TNF activates an IRF1-dependent autocrine loop leading to sustained expression of chemokines and STAT1-dependent type I interferon-response genes. Nat. Immunol. 9, 378–387 (2008).
Menssen, A. et al. SiPaGene: a new repository for instant online retrieval, sharing and meta-analyses of Gene ChIP expression data. BMC Genomics 10, 98 (2009).
Acknowledgements
We thank Mrs X. Sun and M. Jin for technical assistance. This work was supported by the National Natural Science Foundation of China (81788101, 31570871, 31770970), CAMS Innovation Fund for Medical Sciences (2016-12M-1-003) and the National Key Basic Research Program of China (2015CB964403).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Chen, X., Liu, X., Zhang, Y. et al. Methyltransferase Dot1l preferentially promotes innate IL-6 and IFN-β production by mediating H3K79me2/3 methylation in macrophages. Cell Mol Immunol 17, 76–84 (2020). https://doi.org/10.1038/s41423-018-0170-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41423-018-0170-4
Key words
This article is cited by
-
The Mycobacterium tuberculosis methyltransferase Rv2067c manipulates host epigenetic programming to promote its own survival
Nature Communications (2023)
-
Targeting the histone H3 lysine 79 methyltransferase DOT1L in MLL-rearranged leukemias
Journal of Hematology & Oncology (2022)
-
New insights into epigenetic regulation of resistance to PD-1/PD-L1 blockade cancer immunotherapy: mechanisms and therapeutic opportunities
Experimental Hematology & Oncology (2022)
-
Epigenetic regulation of macrophages: from homeostasis maintenance to host defense
Cellular & Molecular Immunology (2020)
-
Targeting the epigenetic regulation of antitumour immunity
Nature Reviews Drug Discovery (2020)