Abstract
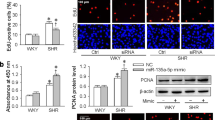
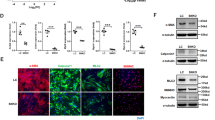
Hypertensive cerebrovascular remodeling involves the enlargement of vascular smooth muscle cells (VSMCs), which activates volume-regulated Cl− channels (VRCCs). The leucine-rich repeat-containing family 8 A (LRRC8A) has been shown to be the molecular identity of VRCCs. However, its role in vascular remodeling during hypertension is unclear. In this study, we used vascular smooth muscle-specific LRRC8A knockout (CKO) mice and an angiotensin II (Ang II)-induced hypertension model. The results showed that cerebrovascular remodeling during hypertension was ameliorated in CKO mice, and extracellular matrix (ECM) deposition was reduced. Based on the RNA-sequencing analysis of aortic tissues, the level of matrix metalloproteinases (MMPs), such as MMP-9 and MMP-14, were reduced in CKO mice with hypertension, which was further verified in vivo by qPCR and immunofluorescence analysis. Knockdown of LRRC8A in VSMCs inhibited the Ang II-induced upregulation of collagen I, fibronectin, and matrix metalloproteinases (MMPs), and overexpression of LRRC8A had the opposite effect. Further experiments revealed an interaction between with-no-lysine (K)-1 (WNK1), which is a “Cl−-sensitive kinase”, and Forkhead transcription factor O3a (FOXO3a), which is a transcription factor that regulates MMP expression. Ang II induced the phosphorylation of WNK1 and downstream FOXO3a, which then increased the expression of MMP-2 and MMP-9. This process was inhibited or potentiated when LRRC8A was knocked down or overexpressed, respectively. Overall, these results demonstrate that LRRC8A knockout in vascular smooth muscle protects against cerebrovascular remodeling during hypertension by reducing ECM deposition and inhibiting the WNK1/FOXO3a/MMP signaling pathway, demonstrating that LRRC8A is a potential therapeutic target for vascular remodeling-associated diseases such as stroke.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Roycik MD, Myers JS, Newcomer RG, Sang QX. Matrix metalloproteinase inhibition in atherosclerosis and stroke. Curr Mol Med. 2013;13:1299–313.
Brown I, Diederich L, Good ME, DeLalio LJ, Murphy SA, Cortese-Krott MM, et al. Vascular smooth muscle remodeling in conductive and resistance arteries in hypertension. Arterioscler Thromb Vasc Biol. 2018;38:1969–85.
Agabiti-Rosei E, Heagerty AM, Rizzoni D. Effects of antihypertensive treatment on small artery remodelling. J Hypertens. 2009;27:1107–14.
Wang X, Khalil RA. Matrix metalloproteinases, vascular remodeling, and vascular disease. Adv Pharmacol. 2018;81:241–330.
Knock GA. NADPH oxidase in the vasculature: Expression, regulation and signalling pathways; role in normal cardiovascular physiology and its dysregulation in hypertension. Free Radic Biol Med. 2019;145:385–427.
Tajsic T, Morrell NW. Smooth muscle cell hypertrophy, proliferation, migration and apoptosis in pulmonary hypertension. Compr Physiol. 2011;1:295–317.
Pasantes-Morales H. Channels and volume changes in the life and death of the cell. Mol Pharmacol. 2016;90:358–70.
Hou L, Liu Y, Sun C, Xu R, Cao G, Wang X. Novel perspective of cardiovascular diseases: volume-regulatory anion channels in the cell membrane. Membranes (Basel). 2022;12:644.
Osei-Owusu J, Yang J, Vitery M, Qiu Z. Molecular biology and physiology of volume-regulated anion channel (VRAC). Curr Top Membr. 2018;81:177–203.
Shi XL, Wang GL, Zhang Z, Liu YJ, Chen JH, Zhou JG, et al. Alteration of volume-regulated chloride movement in rat cerebrovascular smooth muscle cells during hypertension. Hypertension. 2007;49:1371–7.
Chen L, Konig B, Liu T, Pervaiz S, Razzaque YS, Stauber T. More than just a pressure relief valve: physiological roles of volume-regulated LRRC8 anion channels. Biol Chem. 2019;400:1481–96.
Du YH, Guan YY. Chloride channels - new targets for the prevention of stroke. Curr Vasc Pharmacol. 2015;13:441–8.
Shi N, Mei X, Chen SY. Smooth muscle cells in vascular remodeling. Arterioscler Thromb Vasc Biol. 2019;39:e247–e252.
Voss FK, Ullrich F, Munch J, Lazarow K, Lutter D, Mah N, et al. Identification of LRRC8 heteromers as an essential component of the volume-regulated anion channel VRAC. Science. 2014;344:634–8.
Qiu Z, Dubin AE, Mathur J, Tu B, Reddy K, Miraglia LJ, et al. SWELL1, a plasma membrane protein, is an essential component of volume-regulated anion channel. Cell. 2014;157:447–58.
Li XY, Lv XF, Huang CC, Sun L, Ma MM, Liu C, et al. LRRC8A is essential for volume-regulated anion channel in smooth muscle cells contributing to cerebrovascular remodeling during hypertension. Cell Prolif. 2021;54:e13146.
Strange K, Yamada T, Denton JS. A 30-year journey from volume-regulated anion currents to molecular structure of the LRRC8 channel. J Gen Physiol. 2019;151:100–17.
Choi H, Ettinger N, Rohrbough J, Dikalova A, Nguyen HN, Lamb FS. LRRC8A channels support TNFalpha-induced superoxide production by Nox1 which is required for receptor endocytosis. Free Radic Biol Med. 2016;101:413–23.
Lu J, Xu F, Zhang J. Inhibition of angiotensin II-induced cerebrovascular smooth muscle cell proliferation by LRRC8A downregulation through suppressing PI3K/AKT activation. Hum Cell. 2019;32:316–25.
Shekarabi M, Zhang J, Khanna AR, Ellison DH, Delpire E, Kahle KT. WNK kinase signaling in ion homeostasis and human disease. Cell Metab. 2017;25:285–99.
McCormick JA, Ellison DH. The WNKs: atypical protein kinases with pleiotropic actions. Physiol Rev. 2011;91:177–219.
Cabral-Pacheco GA, Garza-Veloz I, Castruita-De LRC, Ramirez-Acuna JM, Perez-Romero BA, Guerrero-Rodriguez JF, et al. The roles of matrix metalloproteinases and their inhibitors in human diseases. Int J Mol Sci. 2020;21:9739.
Ma Z, Mao C, Jia Y, Fu Y, Kong W. Extracellular matrix dynamics in vascular remodeling. Am J Physiol Cell Physiol. 2020;319:C481–C499.
Flamant M, Placier S, Dubroca C, Esposito B, Lopes I, Chatziantoniou C, et al. Role of matrix metalloproteinases in early hypertensive vascular remodeling. Hypertension. 2007;50:212–8.
Wang M, Zhao D, Spinetti G, Zhang J, Jiang LQ, Pintus G, et al. Matrix metalloproteinase 2 activation of transforming growth factor-beta1 (TGF-beta1) and TGF-beta1-type II receptor signaling within the aged arterial wall. Arterioscler Thromb Vasc Biol. 2006;26:1503–9.
Ma MM, Gao M, Guo KM, Wang M, Li XY, Zeng XL, et al. TMEM16A contributes to endothelial dysfunction by facilitating Nox2 NADPH oxidase-derived reactive oxygen species generation in hypertension. Hypertension. 2017;69:892–901.
Gao M, Ma MM, Lu FT, Huang CC, Sun L, Lv XF, et al. Low chloride-regulated ClC-5 contributes to arterial smooth muscle cell proliferation and cerebrovascular remodeling. Hypertension. 2022;79:e73–e85.
Zhang YJ, Zheng HQ, Chen BY, Sun L, Ma MM, Wang GL, et al. WNK1 is required for proliferation induced by hypotonic challenge in rat vascular smooth muscle cells. Acta Pharmacol Sin. 2018;39:35–47.
Tie L, Xiao H, Wu DL, Yang Y, Wang P. A brief guide to good practices in pharmacological experiments: Western blotting. Acta Pharmacol Sin. 2021;42:1015–7.
Pierce BG, Wiehe K, Hwang H, Kim BH, Vreven T, Weng Z. ZDOCK server: interactive docking prediction of protein-protein complexes and symmetric multimers. Bioinformatics. 2014;30:1771–3.
Comeau SR, Gatchell DW, Vajda S, Camacho CJ. ClusPro: a fully automated algorithm for protein-protein docking. Nucleic Acids Res. 2004;32:W96–W99.
Lemarie CA, Tharaux PL, Lehoux S. Extracellular matrix alterations in hypertensive vascular remodeling. J Mol Cell Cardiol. 2010;48:433–9.
Belo VA, Guimaraes DA, Castro MM. Matrix metalloproteinase 2 as a potential mediator of vascular smooth muscle cell migration and chronic vascular remodeling in hypertension. J Vasc Res. 2015;52:221–31.
Calissi G, Lam EW, Link W. Therapeutic strategies targeting FOXO transcription factors. Nat Rev Drug Discov. 2021;20:21–38.
Link W. Introduction to FOXO biology. Methods Mol Biol. 2019;1890:1–9.
Wang C, Wen J, Zhou Y, Li L, Cui X, Wang J, et al. Apelin induces vascular smooth muscle cells migration via a PI3K/Akt/FoxO3a/MMP-2 pathway. Int J Biochem Cell Biol. 2015;69:173–82.
Liu YJ, Wang XG, Tang YB, Chen JH, Lv XF, Zhou JG, et al. Simvastatin ameliorates rat cerebrovascular remodeling during hypertension via inhibition of volume-regulated chloride channel. Hypertension. 2010;56:445–52.
Chen B, Huang C, Lv X, Zheng H, Zhang Y, Sun L, et al. SGK1 mediates the hypotonic protective effect against H2O2-induced apoptosis of rat basilar artery smooth muscle cells by inhibiting the FOXO3a/Bim signaling pathway. Acta Pharmacol Sin. 2020;41:1073–84.
Raffetto JD, Qiao X, Koledova VV, Khalil RA. Prolonged increases in vein wall tension increase matrix metalloproteinases and decrease constriction in rat vena cava: Potential implications in varicose veins. J Vasc Surg. 2008;48:447–56.
Castro MM, Rizzi E, Prado CM, Rossi MA, Tanus-Santos JE, Gerlach RF. Imbalance between matrix metalloproteinases and tissue inhibitor of metalloproteinases in hypertensive vascular remodeling. Matrix Biol. 2010;29:194–201.
Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827–39.
Palei AC, Sandrim VC, Amaral LM, Machado JS, Cavalli RC, Duarte G, et al. Association between matrix metalloproteinase (MMP)-2 polymorphisms and MMP-2 levels in hypertensive disorders of pregnancy. Exp Mol Pathol. 2012;92:217–21.
Derosa G, D’Angelo A, Ciccarelli L, Piccinni MN, Pricolo F, Salvadeo S, et al. Matrix metalloproteinase-2, -9, and tissue inhibitor of metalloproteinase-1 in patients with hypertension. Endothelium. 2006;13:227–31.
Zeng XL, Sun L, Zheng HQ, Wang GL, Du YH, Lv XF, et al. Smooth muscle-specific TMEM16A expression protects against angiotensin II-induced cerebrovascular remodeling via suppressing extracellular matrix deposition. J Mol Cell Cardiol. 2019;134:131–43.
Belo VA, Lacchini R, Miranda JA, Lanna CM, Souza-Costa DC, Tanus-Santos JE. Increased activity of MMP-2 in hypertensive obese children is associated with hypoadiponectinemia. Obes (Silver Spring). 2015;23:177–82.
Onal IK, Altun B, Onal ED, Kirkpantur A, Gul OS, Turgan C. Serum levels of MMP-9 and TIMP-1 in primary hypertension and effect of antihypertensive treatment. Eur J Intern Med. 2009;20:369–72.
Ruddy JM, Akerman AW, Kimbrough D, Nadeau EK, Stroud RE, Mukherjee R, et al. Differential hypertensive protease expression in the thoracic versus abdominal aorta. J Vasc Surg. 2017;66:1543–52.
Zheng LY, Li L, Ma MM, Liu Y, Wang GL, Tang YB, et al. Deficiency of volume-regulated ClC-3 chloride channel attenuates cerebrovascular remodelling in DOCA-salt hypertension. Cardiovasc Res. 2013;100:134–42.
Majumder S, Ren L, Pushpakumar S, Sen U. Hydrogen sulphide mitigates homocysteine-induced apoptosis and matrix remodelling in mesangial cells through Akt/FOXO1 signalling cascade. Cell Signal. 2019;61:66–77.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 82373866 to MM Ma, No. 81773721 to YY Guan, No. 81903598 to XF Lv, No. 82073839 to SJ Liang, No. 82360312 to KM Guo), the Natural Science Foundation of Guangdong Province (2021A1515012427 and 2022A1515012471 to MM Ma, 2021A1515010378 to XF Lv), the Science and Technology Program of Guangzhou (202201020610 to KM Guo), and Fundamental Research Funds for the Central Universities, Sun Yat-Sen University (No. 22ykqb09 to SJ Liang). We would like to thank the personnel at the Advanced Medical Technology Center, The First Affiliated Hospital, Zhongshan School of Medicine, Sun Yat-Sen University for generous support.
Author information
Authors and Affiliations
Contributions
The authors have approved the final article. MMM, XFL, and YYG designed the study. MMM and TC drafted and edited the manuscript. FTL, CCH, and WYL performed most of the experiments. GYY, ZJL, ZYZ, KMG, and YBT assisted with the experimental operations. YC and ZHY assisted with the data collection and statistical analysis. SJL, RPP, and JGZ revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lu, Ft., Huang, Cc., Lai, Wy. et al. Vascular smooth muscle-specific LRRC8A knockout ameliorates angiotensin II-induced cerebrovascular remodeling by inhibiting the WNK1/FOXO3a/MMP signaling pathway. Acta Pharmacol Sin (2024). https://doi.org/10.1038/s41401-024-01280-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41401-024-01280-1