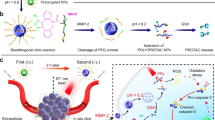
Abstract
Proteolysis targeting chimeras (PROTACs) have emerged as revolutionary anticancer therapeutics that degrade disease-causing proteins. However, the anticancer performance of PROTACs is often impaired by their insufficient bioavailability, unsatisfactory tumor specificity and ability to induce acquired drug resistance. Herein, we propose a polymer-conjugated PROTAC prodrug platform for the tumor-targeted delivery of the most prevalent von Hippel–Lindau (VHL)- and cereblon (CRBN)-based PROTACs, as well as for the precise codelivery of a degrader and conventional small-molecule drugs. The self-assembling PROTAC prodrug nanoparticles (NPs) can specifically target and be activated inside tumor cells to release the free PROTAC for precise protein degradation. The PROTAC prodrug NPs caused more efficient regression of MDA-MB-231 breast tumors in a mouse model by degrading bromodomain-containing protein 4 (BRD4) or cyclin-dependent kinase 9 (CDK9) with decreased systemic toxicity. In addition, we demonstrated that the PROTAC prodrug NPs can serve as a versatile platform for the codelivery of a PROTAC and chemotherapeutics for enhanced anticancer efficiency and combination benefits. This study paves the way for utilizing tumor-targeted protein degradation for precise anticancer therapy and the effective combination treatment of complex diseases.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Sun X, Gao H, Yang Y, He M, Wu Y, Song Y, et al. PROTACs: great opportunities for academia and industry. Signal Transduct Target Ther. 2019;4:64.
Toure M, Crews CM. Small-molecule PROTACS: new approaches to protein degradation. Angew Chem Int Ed Engl. 2016;55:1966–73.
Liu J, Ma J, Liu Y, Xia J, Li Y, Wang ZP, et al. PROTACs: a novel strategy for cancer therapy. Semin Cancer Biol. 2020;67:171–9.
Lai AC, Crews CM. Induced protein degradation: an emerging drug discovery paradigm. Nat Rev Drug Discov. 2017;16:101–14.
Li X, Song Y. Proteolysis-targeting chimera (PROTAC) for targeted protein degradation and cancer therapy. J Hematol Oncol. 2020;13:50.
Burslem GM, Crews CM. Proteolysis-targeting chimeras as therapeutics and tools for biological discovery. Cell. 2020;181:102–14.
Luh LM, Scheib U, Juenemann K, Wortmann L, Brands M, Cromm PM. Prey for the proteasome: targeted protein degradation-a medicinal chemist’s perspective. Angew Chem Int Ed Engl. 2020;59:15448–66.
Popovic D, Vucic D, Dikic I. Ubiquitination in disease pathogenesis and treatment. Nat Med. 2014;20:1242–53.
Chamberlain PP, Hamann LG. Development of targeted protein degradation therapeutics. Nat Chem Biol. 2019;15:937–44.
Dale B, Cheng M, Park KS, Kaniskan HU, Xiong Y, Jin J. Advancing targeted protein degradation for cancer therapy. Nat Rev Cancer. 2021;21:638–54.
Noblejas-Lopez MDM, Nieto-Jimenez C, Burgos M, Gomez-Juarez M, Montero JC, Esparis-Ogando A, et al. Activity of BET-proteolysis targeting chimeric (PROTAC) compounds in triple negative breast cancer. J Exp Clin Cancer Res. 2019;38:383.
Lu J, Qian Y, Altieri M, Dong H, Wang J, Raina K, et al. Hijacking the E3 ubiquitin ligase cereblon to efficiently target BRD4. Chem Biol. 2015;22:755–63.
Minko T. Nanoformulation of BRD4-degrading PROTAC: improving druggability to target the ‘Undruggable’ MYC in pancreatic cancer. Trends Pharmacol Sci. 2020;41:684–6.
Schapira M, Calabrese MF, Bullock AN, Crews CM. Targeted protein degradation: expanding the toolbox. Nat Rev Drug Discov. 2019;18:949–63.
Ocana A, Pandiella A. Proteolysis targeting chimeras (PROTACs) in cancer therapy. J Exp Clin Cancer Res. 2020;39:189.
Moreau K, Coen M, Zhang AX, Pachl F, Castaldi MP, Dahl G, et al. Proteolysis-targeting chimeras in drug development: a safety perspective. Br J Pharmacol. 2020;177:1709–18.
Chen Y, Tandon I, Heelan W, Wang Y, Tang W, Hu Q. Proteolysis-targeting chimera (PROTAC) delivery system: advancing protein degraders towards clinical translation. Chem Soc Rev. 2022;51:5330–50.
Yokoo H, Naito M, Demizu Y. Investigating the cell permeability of proteolysis-targeting chimeras (PROTACs). Expert Opin Drug Discov. 2023;18:357–61.
Li J, Chen X, Lu A, Liang C. Targeted protein degradation in cancers: orthodox PROTACs and beyond. Innovation. 2023;4:100413.
Gao J, Yang L, Lei S, Zhou F, Nie H, Peng B, et al. Stimuli-activatable PROTACs for precise protein degradation and cancer therapy. Sci Bull. 2023;68:1069–85.
Liu J, Chen H, Ma L, He Z, Wang D, Liu Y, et al. Light-induced control of protein destruction by opto-PROTAC. Sci Adv. 2020;6:eaay5154.
Reynders M, Matsuura BS, Berouti M, Simoneschi D, Marzio A, Pagano M, et al. PHOTACs enable optical control of protein degradation. Sci Adv. 2020;6:eaay5064.
Liu J, Chen H, Liu Y, Shen Y, Meng F, Kaniskan HU, et al. Cancer selective target degradation by folate-caged PROTACs. J Am Chem Soc. 2021;143:7380–7.
Dragovich PS, Pillow TH, Blake RA, Sadowsky JD, Adaligil E, Adhikari P, et al. Antibody-mediated delivery of chimeric BRD4 degraders. part 1: exploration of antibody linker, payload loading, and payload molecular properties. J Med Chem. 2021;64:2534–75.
Maneiro MA, Forte N, Shchepinova MM, Kounde CS, Chudasama V, Baker JR, et al. Antibody-PROTAC conjugates enable HER2-dependent targeted protein degradation of BRD4. ACS Chem Biol. 2020;15:1306–12.
He S, Gao F, Ma J, Ma H, Dong G, Sheng C. Aptamer-PROTAC conjugates (APCs) for tumor-specific targeting in breast cancer. Angew Chem Int Ed Engl. 2021;60:23299–305.
Zhang C, Zeng Z, Cui D, He S, Jiang Y, Li J, et al. Semiconducting polymer nano-PROTACs for activatable photo-immunometabolic cancer therapy. Nat Commun. 2021;12:2934.
Zhang C, He S, Zeng Z, Cheng P, Pu K. Smart nano-PROTACs reprogram tumor microenvironment for activatable photo-metabolic cancer immunotherapy. Angew Chem Int Ed Engl. 2022;61:e202114957.
Gao J, Hou B, Zhu Q, Yang L, Jiang X, Zou Z, et al. Engineered bioorthogonal POLY-PROTAC nanoparticles for tumour-specific protein degradation and precise cancer therapy. Nat Commun. 2022;13:4318.
Yang C, Yang Y, Li Y, Ni Q, Li J. Radiotherapy-triggered proteolysis targeting chimera prodrug activation in tumors. J Am Chem Soc. 2023;145:385–91.
Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022;12:31–46.
Narayan RS, Molenaar P, Teng J, Cornelissen FMG, Roelofs I, Menezes R, et al. A cancer drug atlas enables synergistic targeting of independent drug vulnerabilities. Nat Commun. 2020;11:2935.
Jin H, Wang L, Bernards R. Rational combinations of targeted cancer therapies: background, advances and challenges. Nat Rev Drug Discov. 2023;22:213–34.
Vasan N, Baselga J, Hyman DM. A view on drug resistance in cancer. Nature. 2019;575:299–309.
Khan S, Zhang X, Lv D, Zhang Q, He Y, Zhang P, et al. A selective BCL-X(L) PROTAC degrader achieves safe and potent antitumor activity. Nat Med. 2019;25:1938–47.
Flanagan JJ, Qian Y, Gough SM, Andreoli M, Bookbinder M, Cadelina G, et al. Abstract P5-04-18: ARV-471, an oral estrogen receptor PROTAC degrader for breast cancer. Cancer Res. 2021;81:44.
Cao C, Yang J, Chen Y, Zhou P, Wang Y, Du W, et al. Discovery of SK-575 as a highly potent and efficacious proteolysis-targeting chimera degrader of PARP1 for treating cancers. J Med Chem. 2020;63:11012–33.
Jiang B, Gao Y, Che J, Lu W, Kaltheuner IH, Dries R, et al. Discovery and resistance mechanism of a selective CDK12 degrader. Nat Chem Biol. 2021;17:675–83.
Vannam R, Sayilgan J, Ojeda S, Karakyriakou B, Hu E, Kreuzer J, et al. Targeted degradation of the enhancer lysine acetyltransferases CBP and p300. Cell Chem Biol. 2021;28:503–14.e12.
Thummuri D, Khan S, Underwood PW, Zhang P, Wiegand J, Zhang X, et al. Overcoming gemcitabine resistance in pancreatic cancer using the BCL-XL-specific degrader DT2216. Mol Cancer Ther. 2022;21:184–92.
He Y, Ju Y, Hu Y, Wang B, Che S, Jian Y, et al. Brd4 proteolysis-targeting chimera nanoparticles sensitized colorectal cancer chemotherapy. J Control Release. 2023;354:155–66.
Li X, Zhang Z, Gao F, Ma Y, Wei D, Lu Z, et al. c-Myc-targeting PROTAC based on a TNA-DNA bivalent binder for combination therapy of triple-negative breast cancer. J Am Chem Soc. 2023;145:9334–42.
Sun B, Luo C, Yu H, Zhang X, Chen Q, Yang W, et al. Disulfide bond-driven oxidation- and reduction-responsive prodrug nanoassemblies for cancer therapy. Nano Lett. 2018;18:3643–50.
Zhang J, Zhang X, Li Z, Wang Q, Shi Y, Jiang X, et al. The miR-124-3p/neuropilin-1 axis contributes to the proliferation and metastasis of triple-negative breast cancer cells and co-activates the TGF-beta pathway. Front Oncol. 2021;11:654672.
Feng B, Zhou F, Xu Z, Wang T, Wang D, Liu J, et al. Versatile prodrug nanoparticles for acid-triggered precise imaging and organelle-specific combination cancer therapy. Adv Funct Mater. 2016;26:7431–42.
Hou B, Zhou L, Wang H, Saeed M, Wang D, Xu Z, et al. Engineering stimuli-activatable boolean logic prodrug nanoparticles for combination cancer immunotherapy. Adv Mater. 2020;32:e1907210.
Zhou F, Gao J, Tang Y, Zou Z, Jiao S, Zhou Z, et al. Engineering chameleon prodrug nanovesicles to increase antigen presentation and inhibit PD-L1 expression for circumventing immune resistance of cancer. Adv Mater. 2021;33:e2102668.
Zhu Q, Sun F, Li T, Zhou M, Ye J, Ji A, et al. Engineering oxaliplatin prodrug nanoparticles for second near-infrared fluorescence imaging-guided immunotherapy of colorectal cancer. Small. 2021;17:e2007882.
Cao C, He M, Wang L, He Y, Rao Y. Chemistries of bifunctional PROTAC degraders. Chem Soc Rev. 2022;51:7066–114.
Wei D, Wang H, Zeng Q, Wang W, Hao B, Feng X, et al. Discovery of potent and selective CDK9 degraders for targeting transcription regulation in triple-negative breast cancer. J Med Chem. 2021;64:14822–14847.
Xie Z, Hou S, Yang X, Duan Y, Han J, Wang Q, et al. Lessons learned from past cyclin-dependent kinase drug discovery efforts. J Med Chem. 2022;65:6356–89.
Zhou F, Feng B, Wang T, Wang D, Cui Z, Wang S, et al. Theranostic prodrug vesicles for reactive oxygen species-triggered ultrafast drug release and local-regional therapy of metastatic triple-negative breast cancer. Adv Funct Mater. 2017;27:1703674.
Ferrari P, Scatena C, Ghilli M, Bargagna I, Lorenzini G, Nicolini A. Molecular mechanisms, biomarkers and emerging therapies for chemotherapy resistant TNBC. Int J Mol Sci. 2022;23:1665.
Acknowledgements
# These authors contributed equally to this study. Financial supports from the National Natural Science Foundation of China (22074043, U22A20328, 22177120), Science and Technology Commission of Shanghai Municipality (20430711800 and 23ZR1475000), the Sino German Workshop grant (W-0005, LMU/Fudan U) were appreciated. The Mass Spectrometry System and the cell sorter BD Influx of the National Facility for Protein Science in Shanghai (NFPS), Shanghai Advanced Research Institute, CAS are gratefully acknowledged. All animal procedures were carried out under the guidelines approved by the Institutional Animal Care and Use Committee (IACUC) of Shanghai Institute of Materia Medica, CAS.
Author information
Authors and Affiliations
Contributions
ZFZ, LY, HJN, JG: Investigation, Methodology, Formal analysis, and Preparation of the original draft; SML, YL: Investigation; FZ, EW: Writing-review & editing; XHC, ZAX, HJY: Investigation, Methodology, Writing-review & editing, Funding acquisition, Supervision, and Project administration. All authors have approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zou, Zf., Yang, L., Nie, Hj. et al. Tumor-targeted PROTAC prodrug nanoplatform enables precise protein degradation and combination cancer therapy. Acta Pharmacol Sin (2024). https://doi.org/10.1038/s41401-024-01266-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41401-024-01266-z