Abstract
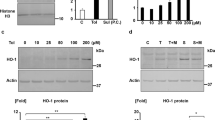
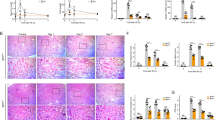
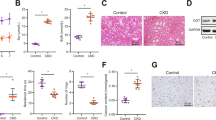
Acute kidney injury (AKI) is often accompanied by uremic encephalopathy resulting from accumulation of uremic toxins in brain possibly due to impaired blood-brain barrier (BBB) function. Anionic uremic toxins are substrates or inhibitors of organic anionic transporters (OATs). In this study we investigated the CNS behaviors and expression/function of BBB OAT3 in AKI rats and mice, which received intraperitoneal injection of cisplatin 8 and 20 mg/kg, respectively. We showed that cisplatin treatment significantly inhibited the expressions of OAT3, synaptophysin and microtubule-associated protein 2 (MAP2), impaired locomotor and exploration activities, and increased accumulation of uremic toxins in the brain of AKI rats and mice. In vitro studies showed that uremic toxins neither alter OAT3 expression in human cerebral microvascular endothelial cells, nor synaptophysin and MAP2 expressions in human neuroblastoma (SH-SY5Y) cells. In contrast, tumour necrosis factor alpha (TNFα) and the conditioned medium (CM) from RAW264.7 cells treated with indoxyl sulfate (IS) significantly impaired OAT3 expression. TNFα and CM from IS-treated BV-2 cells also inhibited synaptophysin and MAP2 expressions in SH-SY5Y cells. The alterations caused by TNFα and CMs in vitro, and by AKI and TNFα in vivo were abolished by infliximab, a monoclonal antibody designed to intercept and neutralize TNFα, suggesting that AKI impaired the expressions of OAT3, synaptophysin and MAP2 in the brain via IS-induced TNFα release from macrophages or microglia (termed as IS-TNFα axis). Treatment of mice with TNFα (0.5 mg·kg−1·d−1, i.p. for 3 days) significantly increased p-p65 expression and reduced the expressions of Nrf2 and HO-1. Inhibiting NF-κB pathway, silencing p65, or activating Nrf2 and HO-1 obviously attenuated TNFα-induced downregulation of OAT3, synaptophysin and MAP2 expressions. Significantly increased p-p65 and decreased Nrf2 and HO-1 protein levels were also detected in brain of AKI mice and rats. We conclude that AKI inhibits the expressions of OAT3, synaptophysin and MAP2 due to IS-induced TNFα release from macrophages or microglia. TNFα impairs the expressions of OAT3, synaptophysin and MAP2 partly via activating NF-κB pathway and inhibiting Nrf2-HO-1 pathway.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout










Similar content being viewed by others
References
Baumgaertel MW, Kraemer M, Berlit P. Neurologic complications of acute and chronic renal disease. Handb Clin Neurol. 2014;119:383–93.
Igiraneza G, Ndayishimiye B, Nkeshimana M, Dusabejambo V, Ogbuagu O. Clinical profile and outcome of patients with acute kidney injury requiring hemodialysis: two years’ experience at a tertiary hospital in Rwanda. BioMed Res Int. 2018;2018:1716420.
Seifter JL, Samuels MA. Uremic encephalopathy and other brain disorders associated with renal failure. Semin Neurol. 2011;31:139–43.
Tanaka S, Okusa MD. Crosstalk between the nervous system and the kidney. Kidney Int. 2020;97:466–76.
Drew DA, Weiner DE, Sarnak MJ. Cognitive impairment in CKD: pathophysiology, management, and prevention. Am J Kidney Dis. 2019;74:782–90.
Kurella Tamura M, Yaffe K. Dementia and cognitive impairment in ESRD: diagnostic and therapeutic strategies. Kidney Int. 2011;79:14–22.
Liu M, Liang Y, Chigurupati S, Lathia JD, Pletnikov M, Sun Z, et al. Acute kidney injury leads to inflammation and functional changes in the brain. J Am Soc Nephrol. 2008;19:1360–70.
Watanabe K, Sato E, Mishima E, Watanabe M, Abe T, Takahashi N, et al. Effect of uremic toxins on hippocampal cell damage: analysis in vitro and in rat model of chronic kidney disease. Heliyon. 2021;7:e06221.
Adachi N, Lei B, Deshpande G, Seyfried FJ, Shimizu I, Nagaro T, et al. Uraemia suppresses central dopaminergic metabolism and impairs motor activity in rats. Intensive Care Med. 2001;27:1655–60.
Bobot M, Thomas L, Moyon A, Fernandez S, McKay N, Balasse L, et al. Uremic toxic blood-brain barrier disruption mediated by AhR activation leads to cognitive impairment during experimental renal dysfunction. J Am Soc Nephrol. 2020;31:1509–21.
Firouzjaei MA, Haghani M, Shid Moosavi SM. Renal ischemia/reperfusion induced learning and memory deficit in the rat: insights into underlying molecular and cellular mechanisms. Brain Res. 2019;1719:263–73.
Vanholder R, De Smet R, Glorieux G, Argilés A, Baurmeister U, Brunet P, et al. Review on uremic toxins: classification, concentration, and interindividual variability. Kidney Int. 2003;63:1934–43.
Vanholder R. Introduction to the toxins special issue on “Novel Issues in Uremic Toxicity”. Toxins. 2018;10:1–9.
Liu X. SLC Family Transporters. Adv Exp Med Biol. 2019;1141:101–202.
Deguchi T, Isozaki K, Yousuke K, Terasaki T, Otagiri M. Involvement of organic anion transporters in the efflux of uremic toxins across the blood-brain barrier. J Neurochem. 2006;96:1051–9.
Okamura T, Okada M, Kikuchi T, Wakizaka H, Zhang MR. Mechanisms of glutathione-conjugate efflux from the brain into blood: involvement of multiple transporters in the course. J Cereb Blood Flow Metab. 2020;40:116–25.
Ohtsuki S, Asaba H, Takanaga H, Deguchi T, Hosoya K, Otagiri M, et al. Role of blood-brain barrier organic anion transporter 3 (OAT3) in the efflux of indoxyl sulfate, a uremic toxin: its involvement in neurotransmitter metabolite clearance from the brain. J Neurochem. 2002;83:57–66.
Ohtsuki S. Physiological function of blood-brain barrier transporters as the CNS supporting and protecting system. Yakugaku zasshi: J Pharma Soc Jpn. 2004;124:791–802.
Grube M, Hagen P, Jedlitschky G. Neurosteroid transport in the brain: role of ABC and SLC transporters. Front Pharmacol. 2018;9:354.
Akanuma S, Uchida Y, Ohtsuki S, Tachikawa M, Terasaki T, Hosoya K. Attenuation of prostaglandin E2 elimination across the mouse blood-brain barrier in lipopolysaccharide-induced inflammation and additive inhibitory effect of cefmetazole. Fluids barriers CNS. 2011;8:24.
Akanuma SI. [Membrane transporters and their regulatory mechanisms at the brain and retinal barriers to establish therapies for refractory central nervous system diseases]. Yakugaku zasshi: J Pharm Soc Jpn. 2020;140:1235–42.
Miyajima M, Kusuhara H, Fujishima M, Adachi Y, Sugiyama Y. Organic anion transporter 3 mediates the efflux transport of an amphipathic organic anion, dehydroepiandrosterone sulfate, across the blood-brain barrier in mice. Drug Metab Dispos. 2011;39:814–9.
Sykes D, Sweet DH, Lowes S, Nigam SK, Pritchard JB, Miller DS. Organic anion transport in choroid plexus from wild-type and organic anion transporter 3 (Slc22a8)-null mice. Am J Physiol Ren Physiol. 2004;286:F972–8.
Hosoya K, Tachikawa M. Roles of organic anion/cation transporters at the blood-brain and blood-cerebrospinal fluid barriers involving uremic toxins. Clin Exp Nephrol. 2011;15:478–85.
De Deyn PP, Marescau B, D’Hooge R, Possemiers I, Nagler J, Mahler C. Guanidino compound levels in brain regions of non-dialyzed uremic patients. Neurochem Int. 1995;27:227–37.
Mair RD, Nguyen H, Huang TT, Plummer NS, Sirich TL, Meyer TW. Accumulation of uremic solutes in the cerebrospinal fluid in experimental acute renal failure. Am J Physiol Ren Physiol. 2019;317:F296–F302.
Erman F, Tuzcu M, Orhan C, Sahin N, Sahin K. Effect of lycopene against cisplatin-induced acute renal injury in rats: organic anion and cation transporters evaluation. Biol trace Elem Res. 2014;158:90–5.
Domitrović R, Cvijanović O, Šušnić V, Katalinić N. Renoprotective mechanisms of chlorogenic acid in cisplatin-induced kidney injury. Toxicology. 2014;324:98–107.
Liu T, Meng Q, Wang C, Liu Q, Guo X, Sun H, et al. Changes in expression of renal Oat1, Oat3 and Mrp2 in cisplatin-induced acute renal failure after treatment of JBP485 in rats. Toxicol Appl Pharmacol. 2012;264:423–30.
Mazumder MK, Giri A, Kumar S, Borah A. A highly reproducible mice model of chronic kidney disease: evidences of behavioural abnormalities and blood-brain barrier disruption. Life Sci. 2016;161:27–36.
Chou AH, Lee CM, Chen CY, Liou JT, Liu FC, Chen YL, et al. Hippocampal transcriptional dysregulation after renal ischemia and reperfusion. Brain Res. 2014;1582:197–210.
Naud J, Laurin LP, Michaud J, Beauchemin S, Leblond FA, Pichette V. Effects of chronic renal failure on brain drug transporters in rats. Drug Metab Dispos. 2012;40:39–46.
Matsushima A, Oda K, Mori N, Murakami T. Modulated function of multidrug resistance-associated proteins in cisplatin-induced acute renal failure rats. Die Pharmazie. 2017;72:209–13.
Kaufmann WE, MacDonald SM, Altamura CR. Dendritic cytoskeletal protein expression in mental retardation: an immunohistochemical study of the neocortex in Rett syndrome. Cereb Cortex. 2000;10:992–1004.
Daftary S, Yon JM, Choi EK, Kim YB, Bice C, Kulikova A, et al. Microtubule associated protein 2 in bipolar depression: impact of pregnenolone. J Affect Disord. 2017;218:49–52.
Ranganathan M, Rahman M, Ganesh S, D’Souza DC, Skosnik PD, Radhakrishnan R, et al. Analysis of circulating exosomes reveals a peripheral signature of astrocytic pathology in schizophrenia. World J Biol Psychiatry. 2022;23:33–45.
DeGiosio RA, Grubisha MJ, MacDonald ML, McKinney BC, Camacho CJ, Sweet RA. More than a marker: potential pathogenic functions of MAP2. Front Mol Neurosci. 2022;15:974890.
Zhang YM, Wei RM, Feng YZ, Zhang KX, Ge YJ, Kong XY, et al. Sleep deprivation aggravates lipopolysaccharide-induced anxiety, depression and cognitive impairment: The role of pro-inflammatory cytokines and synaptic plasticity-associated proteins. J Neuroimmunol. 2023;386:578252.
Shen J, Yang L, Wei W. Role of Fto on CaMKII/CREB signaling pathway of hippocampus in depressive-like behaviors induced by chronic restraint stress mice. Behav Brain Res. 2021;406:113227.
Pang XY, Liu L, Zhang DM, Wang GJ, Xie L, Liu XD. The pharmacokinetics of antofloxacin in renally impaired rats. J Pharm Pharmacol. 2008;60:667–70.
Deng F, Sharma I, Dai Y, Yang M, Kanwar YS. Myo-inositol oxygenase expression profile modulates pathogenic ferroptosis in the renal proximal tubule. J Clin Invest. 2019;129:5033–49.
Wilson CM, Gaber MW, Sabek OM, Zawaski JA, Merchant TE. Radiation-induced astrogliosis and blood-brain barrier damage can be abrogated using anti-TNF treatment. Int J Radiat Oncol, Biol, Phys. 2009;74:934–41.
Kakee A, Takanaga H, Terasaki T, Naito M, Tsuruo T, Sugiyama Y. Efflux of a suppressive neurotransmitter, GABA, across the blood-brain barrier. J Neurochem. 2001;79:110–8.
Lichtenstein L, Ron Y, Kivity S, Ben-Horin S, Israeli E, Fraser GM, et al. Infliximab-related infusion reactions: systematic review. J Crohn’s colitis. 2015;9:806–15.
Cordaro M, Siracusa R, D’Amico R, Genovese T, Franco G, Marino Y, et al. Role of etanercept and infliximab on nociceptive changes induced by the experimental model of fibromyalgia. Int J Mol Sci. 2022;23:6139.
Andrade P, Hoogland G, Del Rosario JS, Steinbusch HW, Visser-Vandewalle V, Daemen MA. Tumor necrosis factor-α inhibitors alleviation of experimentally induced neuropathic pain is associated with modulation of TNF receptor expression. J Neurosci Res. 2014;92:1490–8.
Netrebenko AS, Gureev VV, Pokrovskii MV, Gureeva AV, Tsuverkalova YM, Rozhkov IS. Assessment of the nephroprotective properties of the erythropoietin mimetic peptide and infliximab in kidney ischemia-reperfusion injury in rats. Arch Razi Inst. 2021;76:995–1004.
Ichii O, Otsuka-Kanazawa S, Nakamura T, Ueno M, Kon Y, Chen W, et al. Podocyte injury caused by indoxyl sulfate, a uremic toxin and aryl-hydrocarbon receptor ligand. PLoS One. 2014;9:e108448.
Tan G, Huang C, Chen J, Chen B, Shi Y, Zhi F. An IRF1-dependent pathway of TNFα-induced shedding in intestinal epithelial cells. J Crohns Colitis. 2022;16:133–42.
Xu F, Zhu L, Qian C, Zhou J, Geng D, Li P, et al. Impairment of intestinal monocarboxylate transporter 6 function and expression in diabetic rats induced by combination of high-fat diet and low dose of streptozocin: involvement of butyrate-peroxisome proliferator-activated receptor-γ activation. Drug Metab Dispos. 2019;47:556–66.
Qin YY, Xu P, Wu T, Qian CQ, Fan YL, Gen DH, et al. Bile duct ligation enhances AZT CNS toxicity partly by impairing the expression and function of BCRP in rat brain. Acta Pharmacol Sin. 2020;41:181–91.
Zhao KJ, Chen Y, Hong SJ, Yang YT, Xu J, Yang HY, et al. Characteristics of β-oxidative and reductive metabolism on the acyl side chain of cinnamic acid and its analogues in rats. Acta Pharmacol Sin. 2019;40:1106–18.
Deng L, Zhen Q, Gao J, Jin M, Ding M, Xu B. [Simultaneous determination of plasma indole and skatole in pregnant women with hepatitis B virus infection by high performance liquid chromatography]. Se pu = Chin J Chromatogr. 2017;35:735–40.
Kong W, Sun X, Yu S, Liu P, Zheng X, Zhang J, et al. Bile duct ligation increased dopamine levels in the cerebral cortex of rats partly due to induction of tyrosine hydroxylase. Br J Pharmacol. 2023;180:1690–709.
Bao Y, Luchetti D, Schaeffer E, Cutrone J. Determination of kynurenic acid in rat cerebrospinal fluid by HPLC with fluorescence detection. Biomed Chromatogr. 2016;30:62–7.
Zhang L, Liu XD, Xie L, Wang GJ. P-glycoprotein restricted transport of nimodipine across blood-brain barrier. Acta Pharmacol Sin. 2003;24:903–6.
Ferrari D, Speciale A, Cristani M, Fratantonio D, Molonia MS, Ranaldi G, et al. Cyanidin-3-O-glucoside inhibits NF-κB signalling in intestinal epithelial cells exposed to TNF-α and exerts protective effects via Nrf2 pathway activation. Toxicol Lett. 2016;264:51–8.
Chen T, Zhang X, Zhu G, Liu H, Chen J, Wang Y, et al. Quercetin inhibits TNF-α induced HUVECs apoptosis and inflammation via downregulating NF-κB and AP-1 signaling pathway in vitro. Medicine. 2020;99:e22241.
El-Shitany NA, Eid BG. Icariin modulates carrageenan-induced acute inflammation through HO-1/Nrf2 and NF-κB signaling pathways. Biomed Pharmacother. 2019;120:109567.
Zhao C, Xiao C, Feng S, Bai J. Artemisitene alters LPS-induced oxidative stress, inflammation and ferroptosis in liver through Nrf2/HO-1 and NF-κB pathway. Front Pharmacol. 2023;14:1177542.
Wang S, Avery JE, Hannafon BN, Lind SE, Ding WQ. Zinc protoporphyrin suppresses cancer cell viability through a heme oxygenase-1-independent mechanism: the involvement of the Wnt/β-catenin signaling pathway. Biochem Pharmacol. 2013;85:1611–8.
Idriss HT, Naismith JH. TNF alpha and the TNF receptor superfamily: structure-function relationship(s). Microsc Res Tech. 2000;50:184–95.
Facchin BM, Dos Reis GO, Vieira GN, Mohr ETB, da Rosa JS, Kretzer IF, et al. Inflammatory biomarkers on an LPS-induced RAW 264.7 cell model: a systematic review and meta-analysis. Inflamm Res. 2022;71:741–58.
Yao K, Zhao YF. Aging modulates microglia phenotypes in neuroinflammation of MPTP-PD mice. Exp Gerontol. 2018;111:86–93.
Ji C, Luo Y, Zou C, Huang L, Tian R, Lu Z. Effect of astragaloside IV on indoxyl sulfate-induced kidney injury in mice via attenuation of oxidative stress. BMC Pharmacol Toxicol. 2018;19:53.
Lizarazo DA, Lizarazo J. Uremic encephalopathy. Radiology. 2023;307:e221602.
Biasioli S, D’Andrea G, Feriani M, Chiaramonte S, Fabris A, Ronco C, et al. Uremic encephalopathy: an updating. Clin Nephrol. 1986;25:57–63.
Kang A, Arnold R, Gallagher M, Snelling P, Green J, Fernando M, et al. Effect of hemodiafiltration on the progression of neuropathy with kidney failure: a randomized controlled trial. Clin J Am Soc Nephrol. 2021;16:1365–75.
Favretto G, Souza LM, Gregório PC, Cunha RS, Maciel RAP, Sassaki GL, et al. Role of organic anion transporters in the uptake of protein-bound uremic toxins by human endothelial cells and monocyte chemoattractant protein-1 expression. J Vasc Res. 2017;54:170–9.
Watanabe H, Sakaguchi Y, Sugimoto R, Kaneko K, Iwata H, Kotani S, et al. Human organic anion transporters function as a high-capacity transporter for p-cresyl sulfate, a uremic toxin. Clin Exp Nephrol. 2014;18:814–20.
Uwai Y, Honjo H, Iwamoto K. Interaction and transport of kynurenic acid via human organic anion transporters hOAT1 and hOAT3. Pharmacol Res. 2012;65:254–60.
Donovan MD, Schellekens H, Boylan GB, Cryan JF, Griffin BT. In vitro bidirectional permeability studies identify pharmacokinetic limitations of NKCC1 inhibitor bumetanide. Eur J Pharmacol. 2016;770:117–25.
Li Y, Yan J, Wang M, Lv J, Yan F, Chen J. Uremic toxin indoxyl sulfate promotes proinflammatory macrophage activation by regulation of β-catenin and YAP pathways. J Mol Histol. 2021;52:197–205.
Lv J, Chen J, Wang M, Yan F. Klotho alleviates indoxyl sulfate-induced heart failure and kidney damage by promoting M2 macrophage polarization. Aging. 2020;12:9139–50.
Adesso S, Popolo A, Bianco G, Sorrentino R, Pinto A, Autore G, et al. The uremic toxin indoxyl sulphate enhances macrophage response to LPS. PLoS One. 2013;8:e76778.
Kim HY, Yoo TH, Cho JY, Kim HC, Lee WW. Indoxyl sulfate-induced TNF-α is regulated by crosstalk between the aryl hydrocarbon receptor, NF-κB, and SOCS2 in human macrophages. FASEB J. 2019;33:10844–58.
Qoreishi M, Panahi M, Dorodi O, Ghanbari N, Jousheghan SS. Involvement of NF-κB/NLRP3 axis in the progression of aseptic loosening of total joint arthroplasties: a review of molecular mechanisms. Naunyn-Schmiedeberg’s Arch Pharmacol. 2022;395:757–67.
Kim HY, Yoo TH, Hwang Y, Lee GH, Kim B, Jang J, et al. Indoxyl sulfate (IS)-mediated immune dysfunction provokes endothelial damage in patients with end-stage renal disease (ESRD). Sci Rep. 2017;7:3057.
Climaco-Arvizu S, Domínguez-Acosta O, Cabañas-Cortés MA, Rodríguez-Sosa M, Gonzalez FJ, Vega L, et al. Aryl hydrocarbon receptor influences nitric oxide and arginine production and alters M1/M2 macrophage polarization. Life Sci. 2016;155:76–84.
Riemschneider S, Kohlschmidt J, Fueldner C, Esser C, Hauschildt S, Lehmann J. Aryl hydrocarbon receptor activation by benzo(a)pyrene inhibits proliferation of myeloid precursor cells and alters the differentiation state as well as the functional phenotype of murine bone marrow-derived macrophages. Toxicol Lett. 2018;296:106–13.
Li X, Li N, Han Y, Rao K, Ji X, Ma M. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD)-induced suppression of immunity in THP-1-derived macrophages and the possible mechanisms. Environ Pollut (Barking, Essex: 1987). 2021;287:117302.
Sivandzade F, Prasad S, Bhalerao A, Cucullo L. NRF2 and NF-κB interplay in cerebrovascular and neurodegenerative disorders: molecular mechanisms and possible therapeutic approaches. Redox Biol. 2019;21:101059.
Velagapudi R, Kumar A, Bhatia HS, El-Bakoush A, Lepiarz I, Fiebich BL, et al. Inhibition of neuroinflammation by thymoquinone requires activation of Nrf2/ARE signalling. Int Immunopharmacol. 2017;48:17–29.
Burckhardt BC, Henjakovic M, Hagos Y, Burckhardt G. Counter-flow suggests transport of dantrolene and 5-OH dantrolene by the organic anion transporters 2 (OAT2) and 3 (OAT3). Pflug Arch: Eur J Physiol. 2016;468:1909–18.
Harper CB, Blumrich EM, Cousin MA. Synaptophysin controls synaptobrevin-II retrieval via a cryptic C-terminal interaction site. J Biol Chem. 2021;296:100266.
Cousin MA. Synaptophysin-dependent synaptobrevin-2 trafficking at the presynapse-mechanism and function. J Neurochem. 2021;159:78–89.
Araki T, Ikegaya Y, Koyama R. The effects of microglia- and astrocyte-derived factors on neurogenesis in health and disease. Eur J Neurosci. 2021;54:5880–901.
Smith JA, Das A, Ray SK, Banik NL. Role of pro-inflammatory cytokines released from microglia in neurodegenerative diseases. Brain Res Bull. 2012;87:10–20.
Zhao SC, Ma LS, Chu ZH, Xu H, Wu WQ, Liu F. Regulation of microglial activation in stroke. Acta Pharmacol Sin. 2017;38:445–58.
Kaur D, Sharma V, Deshmukh R. Activation of microglia and astrocytes: a roadway to neuroinflammation and Alzheimer’s disease. Inflammopharmacology. 2019;27:663–77.
Lee B, Ines I, Je J, Park EJ, Seong H, Jo MG, et al. Effect of renal ischemia reperfusion on brain neuroinflammation. Biomedicines. 2022;10:2993.
Li LC, Chen WY, Chen JB, Lee WC, Chang CC, Tzeng HT, et al. The AST-120 recovers uremic toxin-induced cognitive deficit via NLRP3 inflammasome pathway in astrocytes and microglia. Biomedicines. 2021;9:1252.
Martín-de-Saavedra MD, Budni J, Cunha MP, Gómez-Rangel V, Lorrio S, Del Barrio L, et al. Nrf2 participates in depressive disorders through an anti-inflammatory mechanism. Psychoneuroendocrinology. 2013;38:2010–22.
Zhao J, Bi W, Xiao S, Lan X, Cheng X, Zhang J, et al. Neuroinflammation induced by lipopolysaccharide causes cognitive impairment in mice. Sci Rep. 2019;9:5790.
Velagapudi R, El-Bakoush A, Olajide OA. Activation of Nrf2 pathway contributes to neuroprotection by the dietary flavonoid tiliroside. Mol Neurobiol. 2018;55:8103–23.
Pan W, Zadina JE, Harlan RE, Weber JT, Banks WA, Kastin AJ. Tumor necrosis factor-alpha: a neuromodulator in the CNS. Neurosci Biobehav Rev. 1997;21:603–13.
Pan W, Cornélissen G, Halberg F, Kastin AJ. Selected contribution: circadian rhythm of tumor necrosis factor-alpha uptake into mouse spinal cord. J Appl Physiol (Bethesda, Md: 1985). 2002;92:1357–62.
Holditch SJ, Brown CN, Lombardi AM, Nguyen KN, Edelstein CL. Recent advances in models, mechanisms, biomarkers, and interventions in cisplatin-induced acute kidney injury. Int J Mol Sci. 2019;20:1–25.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 82373943, 82173844, 82204511, and 82073922) and the Jiangsu Funding Program for Excellent Postdoctoral Talent (No. 1412200067).
Author information
Authors and Affiliations
Contributions
LL, XDL, LJ carried out the ideas and designed the studies; LJ, XYS, SQW, YLL, LJL, HZ, ZYW conducted the experiments, acquired the data and documented data analysis; XYS, WHW interpreted results and plotted figures; LJ, LL, XDL drafted, revised and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jiang, L., Sun, Xy., Wang, Sq. et al. Indoxyl sulphate-TNFα axis mediates uremic encephalopathy in rodent acute kidney injury. Acta Pharmacol Sin (2024). https://doi.org/10.1038/s41401-024-01251-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41401-024-01251-6