Abstract
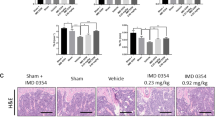
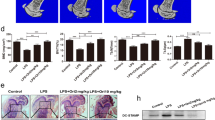
Osteoporosis results from overactivation of osteoclasts. There are currently few drug options for treatment of this disease. Since the successful development of allosteric inhibitors, phosphatases have become attractive therapeutic targets. Protein phosphatase 1, regulatory subunit 15 A (PPP1R15A), is a stress-responsive protein, which promotes the UPR (unfolded protein response) and restores protein homeostasis. In this study we investigated the role of PPP1R15A in osteoporosis and osteoclastogenesis. Ovariectomy (OVX)-induced osteoporosis mouse model was established, osteoporosis was evaluated in the left femurs using micro-CT. RANKL-stimulated osteoclastogenesis was used as in vitro models. We showed that PPP1R15A expression was markedly increased in BMMs derived from OVX mice and during RANKL-induced osteoclastogenesis in vitro. Knockdown of PPP1R15A or application of Sephin1 (a PPP1R15A allosteric inhibitor in a phase II clinical trial) significantly inhibited osteoclastogenesis in vitro. Sephin1 (0.78, 3.125 and 12.5 μM) dose-dependently mitigated the changes in NF-κB, MAPK, and c-FOS and the subsequent nuclear factor of activated T cells 1 (NFATc1) translocation in RANKL-stimulated BMMs. Both Sephin1 and PPP1R15A knockdown increased the phosphorylated form of eukaryotic initiation factor 2α (eIF2α); knockdown of eIF2α reduced the inhibitory effects of Sephin1 on NFATc1-luc transcription and osteoclast formation. Furthermore, Sephin1 or PPP1R15A knockdown suppressed osteoclastogenesis in CD14+ monocytes from osteoporosis patients. In OVX mice, injection of Sephin1 (4, 8 mg/kg, i.p.) every two days for 6 weeks significantly inhibited bone loss, and restored bone destruction and decreased TRAP-positive cells. This study has identified PPP1R15A as a novel target for osteoclast differentiation, and genetic inhibition or allosteric inhibitors of PPP1R15A, such as Sephin1, can be used to treat osteoporosis.

This study revealed that PPP1R15A expression was increased in osteoporosis in both human and mice. Inhibition of PPP1R15A by specific knockdown or an allosteric inhibitor Sephin1 mitigated murine osteoclast formation in vitro and attenuated ovariectomy-induced osteoporosis in vivo. PPP1R15A inhibition also suppressed pathogenic osteoclastogenesis in CD14+ monocytes from osteoporosis patients. These results identify PPP1R15A as a novel regulator of osteoclastogenesis and a valuable therapeutic target for osteoporosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Ukon Y, Makino T, Kodama J, Tsukazaki H, Tateiwa D, Yoshikawa H, et al. Molecular-based treatment strategies for osteoporosis: a literature review. Int J Mol Sci. 2019;20:2557.
Noh JY, Yang Y, Jung H. Molecular mechanisms and emerging therapeutics for osteoporosis. Int J Mol Sci. 2020;21:7623.
Dömötör ZR, Vörhendi N, Hanák L, Hegyi P, Kiss S, Csiki E, et al. Oral treatment with bisphosphonates of osteoporosis does not increase the risk of severe gastrointestinal side effects: a meta-analysis of randomized controlled trials. Front Endocrinol. 2020;11:573976.
Levin VA, Jiang X, Kagan R. Estrogen therapy for osteoporosis in the modern era. Osteoporos Int. 2018;29:1049–55.
Khosla S, Hofbauer LC. Osteoporosis treatment: recent developments and ongoing challenges. Lancet Diabetes Endocrinol. 2017;5:898–907.
Lamy O, Stoll D, Aubry-Rozier B, Rodriguez EG. Stopping Denosumab. Curr Osteoporos Rep. 2019;17:8–15.
Liu FC, Luk KC, Chen YC. Risk comparison of osteonecrosis of the jaw in osteoporotic patients treated with bisphosphonates vs. denosumab: a multi-institutional retrospective cohort study in Taiwan. Osteoporos Int. 2023;34:1729–37.
Ono T, Nakashima T. Recent advances in osteoclast biology. Histochem Cell Biol. 2018;149:325–41.
Xiao L, Zhong M, Huang Y, Zhu J, Tang W, Li D, et al. Puerarin alleviates osteoporosis in the ovariectomy-induced mice by suppressing osteoclastogenesis via inhibition of TRAF6/ROS-dependent MAPK/NF-κB signaling pathways. Aging. 2020;12:21706–29.
Liu Y, Wang C, Wang G, Sun Y, Deng Z, Chen L, et al. Loureirin B suppresses RANKL-induced osteoclastogenesis and ovariectomized osteoporosis via attenuating NFATc1 and ROS activities. Theranostics. 2019;9:4648–62.
Negishi-Koga T, Takayanagi H. Ca2+-NFATc1 signaling is an essential axis of osteoclast differentiation. Immunol Rev. 2009;231:241–56.
Vainonen JP, Momeny M, Westermarck J. Druggable cancer phosphatases. Sci Transl Med. 2021;13:eabe2967.
Krzyzosiak A, Sigurdardottir A, Luh L, Carrara M, Das I, Schneider K, et al. Target-based discovery of an inhibitor of the regulatory phosphatase PPP1R15B. Cell. 2018;174:1216–28.e1219.
Mullard A. Phosphatases start shedding their stigma of undruggability. Nat Rev Drug Discov. 2018;17:847–9.
LaMarche MJ, Acker M, Argintaru A, Bauer D, Boisclair J, Chan H, et al. Identification of TNO155, an allosteric SHP2 inhibitor for the treatment of cancer. J Med Chem. 2020;63:13578–94.
Das I, Krzyzosiak A, Schneider K, Wrabetz L, D’Antonio M, Barry N, et al. Preventing proteostasis diseases by selective inhibition of a phosphatase regulatory subunit. Science. 2015;348:239–42.
Chen Y, Podojil JR, Kunjamma RB, Jones J, Weiner M, Lin W, et al. Sephin1, which prolongs the integrated stress response, is a promising therapeutic for multiple sclerosis. Brain. 2019;142:344–61.
Thapa S, Abdelaziz DH, Abdulrahman BA, Schatzl HM. Sephin1 reduces prion infection in prion-infected cells and animal model. Mol Neurobiol. 2020;57:2206–19.
Harding HP, Calfon M, Urano F, Novoa I, Ron D. Transcriptional and translational control in the mammalian unfolded protein response. Annu Rev Cell Dev Biol. 2002;18:575–99.
Ma T, Trinh MA, Wexler AJ, Bourbon C, Gatti E, Pierre P, et al. Suppression of eIF2α kinases alleviates Alzheimer’s disease-related plasticity and memory deficits. Nat Neurosci. 2013;16:1299–305.
Harding HP, Zeng H, Zhang Y, Jungries R, Chung P, Plesken H, et al. Diabetes mellitus and exocrine pancreatic dysfunction in Perk-/- mice reveals a role for translational control in secretory cell survival. Mol Cell. 2001;7:1153–63.
Sun X, Aimé P, Dai D, Ramalingam N, Crary JF, Burke RE, et al. Guanabenz promotes neuronal survival via enhancement of ATF4 and parkin expression in models of Parkinson disease. Exp Neurol. 2018;303:95–107.
Zou B, Zheng J, Deng W, Tan Y, Jie L, Qu Y, et al. Kirenol inhibits RANKL-induced osteoclastogenesis and prevents ovariectomized-induced osteoporosis via suppressing the Ca2+-NFATc1 and Cav-1 signaling pathways. Phytomedicine. 2021;80:153377.
Wang C, Steer JH, Joyce DA, Yip KH, Zheng MH, Xu J. 12-O-tetradecanoylphorbol-13-acetate (TPA) inhibits osteoclastogenesis by suppressing RANKL-induced NF-kappaB activation. J Bone Min Res. 2003;18:2159–68.
Zhou L, Liu Q, Yang M, Wang T, Yao J, Cheng J, et al. Dihydroartemisinin, an anti-Malaria drug, suppresses estrogen deficiency-induced osteoporosis, osteoclast formation, and RANKL-induced signaling pathways. J Bone Min Res. 2016;31:964–74.
Zalli D, Neff L, Nagano K, Shin NY, Witke W, Gori F, et al. The actin-binding protein cofilin and its interaction with cortactin are required for podosome patterning in osteoclasts and bone resorption in vivo and in vitro. J Bone Min Res. 2016;31:1701–12.
Abu-Amer Y. NF-κB signaling and bone resorption. Osteoporos Int. 2013;24:2377–86.
Pardo VG, Boland R, de Boland AR. 1alpha,25(OH)2-Vitamin D3 stimulates intestinal cell p38 MAPK activity and increases c-Fos expression. Int J Biochem Cell Biol. 2006;38:1181–90.
Carrara M, Sigurdardottir A, Bertolotti A. Decoding the selectivity of eIF2α holophosphatases and PPP1R15A inhibitors. Nat Struct Mol Biol. 2017;24:708–16.
Fusade-Boyer M, Dupré G, Bessière P, Khiar S, Quentin-Froignant C, Beck C, et al. Evaluation of the antiviral activity of Sephin1 treatment and its consequences on eIF2α phosphorylation in response to viral infections. Front Immunol. 2019;10:134.
Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet. 2011;377:1276–87.
Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25:2359–81.
Blangy A, Bompard G, Guerit D, Marie P, Maurin J, Morel A, et al. The osteoclast cytoskeleton—current understanding and therapeutic perspectives for osteoporosis. J Cell Sci. 2020;133:jcs244798.
Cheng C, Wentworth K, Shoback DM. New frontiers in osteoporosis therapy. Annu Rev Med. 2020;71:277–88.
Querrer R, Ferrare N, Melo N, Stefani CM, Dos Reis PED, Mesquita CRM, et al. Differences between bisphosphonate-related and denosumab-related osteonecrosis of the jaws: a systematic review. Support Care Cancer: Off J Multinatl Assoc Support Care Cancer. 2021;29:2811–20.
Madeira M, Rocha AC, Moreira CA, Aguiar ÁMM, Maeda SS, Cardoso AS, et al. Prevention and treatment of oral adverse effects of antiresorptive medications for osteoporosis - a position paper of the Brazilian society of endocrinology and metabolism (SBEM), Brazilian society of stomatology and oral pathology (Sobep), and Brazilian association for bone evaluation and osteometabolism (Abrasso). Arch Endocrinol Metab. 2021;64:664–72.
Ferrari S, Lewiecki EM, Butler PW, Kendler DL, Napoli N, Huang S, et al. Favorable skeletal benefit/risk of long-term denosumab therapy: a virtual-twin analysis of fractures prevented relative to skeletal safety events observed. Bone. 2020;134:115287.
Hollander MC, Sheikh MS, Yu K, Zhan Q, Iglesias M, Woodworth C, et al. Activation of Gadd34 by diverse apoptotic signals and suppression of its growth inhibitory effects by apoptotic inhibitors. Int J cancer. 2001;96:22–31.
Zheng C, Wang C, Jie Q, Yang L. Analyze Mouse knockout models of UPR pathway elements. Methods Mol Biol. 2022;2378:205–19.
Briggs MD, Dennis EP, Dietmar HF, Pirog KA. New developments in chondrocyte ER stress and related diseases. F1000Res. 2020;9:F1000.
Marciniak SJ, Chambers JE, Ron D. Pharmacological targeting of endoplasmic reticulum stress in disease. Nat Rev Drug Discov. 2022;21:115–40.
Zhao Q, Wang X, Liu Y, He A, Jia R. NFATc1: functions in osteoclasts. Int J Biochem Cell Biol. 2010;42:576–79.
Sitara D, Aliprantis AO. Transcriptional regulation of bone and joint remodeling by NFAT. Immunol Rev. 2010;233:286–300.
Kittaka M, Mayahara K, Mukai T, Yoshimoto T, Yoshitaka T, Gorski JP, et al. Cherubism mice also deficient in c-Fos exhibit inflammatory bone destruction executed by macrophages that express MMP14 despite the absence of TRAP+ osteoclasts. J Bone Min Res. 2018;33:167–81.
Li J, Li X, Liu D, Hamamura K, Wan Q, Na S, et al. eIF2α signaling regulates autophagy of osteoblasts and the development of osteoclasts in OVX mice. Cell Death Dis. 2019;10:921.
Kitamura M. Biphasic, bidirectional regulation of NF-kappaB by endoplasmic reticulum stress. Antioxid Redox Signal. 2009;11:2353–64.
Ruiz A, Zuazo J, Ortiz-Sanz C, Luchena C, Matute C, Alberdi E. Sephin1 protects neurons against excitotoxicity independently of the integrated stress response. Int J Mol Sci. 2020;21:6088.
Callejo G, Pattison LA, Greenhalgh JC, Chakrabarti S, Andreopoulou E, Hockley JRF, et al. In silico screening of GMQ-like compounds reveals guanabenz and Sephin1 as new allosteric modulators of acid-sensing ion channel 3. Biochem Pharmacol. 2020;174:113834.
Way SW, Podojil JR, Clayton BL, Zaremba A, Collins TL, Kunjamma RB, et al. Pharmaceutical integrated stress response enhancement protects oligodendrocytes and provides a potential multiple sclerosis therapeutic. Nat Commun. 2015;6:6532.
Tsaytler P, Harding HP, Ron D, Bertolotti A. Selective inhibition of a regulatory subunit of protein phosphatase 1 restores proteostasis. Science. 2011;332:91–94.
Teng Y, Gao M, Wang J, Kong Q, Hua H, Luo T, et al. Inhibition of eIF2α dephosphorylation enhances TRAIL-induced apoptosis in hepatoma cells. Cell Death Dis. 2014;5:e1060.
Zhong M, Wu Z, Chen Z, Ren Q, Zhou J. Advances in the interaction between endoplasmic reticulum stress and osteoporosis. Biomed Pharmacother. 2023;165:115134.
Huang W, Gong Y, Yan L. ER stress, the unfolded protein response and osteoclastogenesis: a review. Biomolecules. 2023;13:1050.
Guo J, Ren R, Sun K, Yao X, Lin J, Wang G, et al. PERK controls bone homeostasis through the regulation of osteoclast differentiation and function. Cell Death Dis. 2020;11:847.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (82173825, 81773740, and 81972064) and the Science and Technology Plan Project of Guangzhou City (201804010027).
Author information
Authors and Affiliations
Contributions
This study was designed by ZBD, YC, XJL, and conducted by ZBD, YC, YRZ, YYW, QY, JHZ, WDD, ZYC, and LHL. The manuscript was written by ZBD and YC, and revised by HJ and XJL.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ding, Zb., Chen, Y., Zheng, Yr. et al. Inhibition of PPP1R15A alleviates osteoporosis via suppressing RANKL-induced osteoclastogenesis. Acta Pharmacol Sin 45, 790–802 (2024). https://doi.org/10.1038/s41401-023-01209-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-023-01209-0