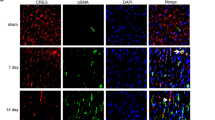
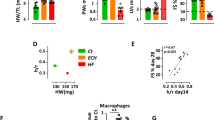
Abstract
Stimulation of adult cardiomyocyte proliferation is a promising strategy for treating myocardial infarction (MI). Earlier studies have shown increased CCL2 levels in plasma and cardiac tissue both in MI patients and mouse models. In present study we investigated the role of CCL2 in cardiac regeneration and the underlying mechanisms. MI was induced in adult mice by permanent ligation of the left anterior descending artery, we showed that the serum and cardiac CCL2 levels were significantly increased in MI mice. Intramyocardial injection of recombinant CCL2 (rCCL2, 1 μg) immediately after the surgery significantly promoted cardiomyocyte proliferation, improved survival rate and cardiac function, and diminished scar sizes in post-MI mice. Alongside these beneficial effects, we observed an increased angiogenesis and decreased cardiomyocyte apoptosis in post-MI mice. Conversely, treatment with a selective CCL2 synthesis inhibitor Bindarit (30 μM) suppressed both CCL2 expression and cardiomyocyte proliferation in P1 neonatal rat ventricle myocytes (NRVMs). We demonstrated in NRVMs that the CCL2 stimulated cardiomyocyte proliferation through STAT3 signaling: treatment with rCCL2 (100 ng/mL) significantly increased the phosphorylation levels of STAT3, whereas a STAT3 phosphorylation inhibitor Stattic (30 μM) suppressed rCCL2-induced cardiomyocyte proliferation. In conclusion, this study suggests that CCL2 promotes cardiac regeneration via activation of STAT3 signaling, underscoring its potential as a therapeutic agent for managing MI and associated heart failure.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Reed GW, Rossi JE, Cannon CP. Acute myocardial infarction. Lancet. 2017;389:197–210.
Chablais F, Veit J, Rainer G, Jazwinska A. The zebrafish heart regenerates after cryoinjury-induced myocardial infarction. BMC Dev Biol. 2011;11:21.
Wang Z, Cui M, Shah AM, Ye W, Tan W, Min YL, et al. Mechanistic basis of neonatal heart regeneration revealed by transcriptome and histone modification profiling. Proc Natl Acad Sci USA. 2019;116:18455–65.
Uygur A, Lee RT. Mechanisms of cardiac regeneration. Dev Cell. 2016;36:362–74.
Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102.
Yuan X, Braun T. Multimodal regulation of cardiac myocyte proliferation. Circ Res. 2017;121:293–309.
Aurora AB, Porrello ER, Tan W, Mahmoud AI, Hill JA, Bassel-Duby R, et al. Macrophages are required for neonatal heart regeneration. J Clin Invest. 2014;124:1382–92.
Singh S, Anshita D, Ravichandiran V. MCP-1: function, regulation, and involvement in disease. Int Immunopharmacol. 2021;101:107598.
Chen B, Frangogiannis NG. Chemokines in myocardial infarction. J Cardiovasc Transl Res. 2021;14:35–52.
de Lemos JA, Morrow DA, Sabatine MS, Murphy SA, Gibson CM, Antman EM, et al. Association between plasma levels of monocyte chemoattractant protein-1 and long-term clinical outcomes in patients with acute coronary syndromes. Circulation. 2003;107:690–5.
Zhu Y, Hu C, Du Y, Zhang J, Liu J, Han H, et al. Significant association between admission serum monocyte chemoattractant protein-1 and early changes in myocardial function in patients with first ST-segment elevation myocardial infarction after primary percutaneous coronary intervention. BMC Cardiovasc Disord. 2019;19:107.
Morimoto H, Takahashi M, Izawa A, Ise H, Hongo M, Kolattukudy PE, et al. Cardiac overexpression of monocyte chemoattractant protein-1 in transgenic mice prevents cardiac dysfunction and remodeling after myocardial infarction. Circ Res. 2006;99:891–9.
Quaife-Ryan GA, Sim CB, Ziemann M, Kaspi A, Rafehi H, Ramialison M, et al. Multicellular transcriptional analysis of mammalian heart regeneration. Circulation. 2017;136:1123–39.
Whitehead AJ, Engler AJ. Regenerative cross talk between cardiac cells and macrophages. Am J Physiol Heart Circ Physiol. 2021;320:H2211–H21.
Qian Y, Ding P, Xu J, Nie X, Lu B. CCL2 activates AKT signaling to promote glycolysis and chemoresistance in glioma cells. Cell Biol Int. 2022;46:819–28.
Keeley EC, Mehrad B, Strieter RM. Chemokines as mediators of neovascularization. Arterioscler Thromb Vasc Biol. 2008;28:1928–36.
Li X, Sun X, Carmeliet P. Hallmarks of endothelial cell metabolism in health and disease. Cell Metab. 2019;30:414–33.
Ge S, Shrestha B, Paul D, Keating C, Cone R, Guglielmotti A, et al. The CCL2 synthesis inhibitor bindarit targets cells of the neurovascular unit, and suppresses experimental autoimmune encephalomyelitis. J Neuroinflammation. 2012;9:171.
Tian DS, Peng J, Murugan M, Feng LJ, Liu JL, Eyo UB, et al. Chemokine CCL2-CCR2 signaling induces neuronal cell death via STAT3 activation and IL-1beta production after status epilepticus. J Neurosci. 2017;37:7878–92.
Yao M, Fang W, Smart C, Hu Q, Huang S, Alvarez N, et al. CCR2 chemokine receptors enhance growth and cell-cycle progression of breast cancer cells through SRC and PKC activation. Mol Cancer Res. 2019;17:604–17.
Fang Y, Gupta V, Karra R, Holdway JE, Kikuchi K, Poss KD. Translational profiling of cardiomyocytes identifies an early Jak1/Stat3 injury response required for zebrafish heart regeneration. Proc Natl Acad Sci USA. 2013;110:13416–21.
Uchihara Y, Ohe T, Mashino T, Kidokoro T, Tago K, Tamura H, et al. N-Acetyl cysteine prevents activities of STAT3 inhibitors, Stattic and BP-1-102 independently of its antioxidant properties. Pharmacol Rep. 2019;71:1067–78.
Eschenhagen T, Bolli R, Braun T, Field LJ, Fleischmann BK, Frisen J, et al. Cardiomyocyte regeneration: a consensus statement. Circulation. 2017;136:680–6.
Senyo SE, Steinhauser ML, Pizzimenti CL, Yang VK, Cai L, Wang M, et al. Mammalian heart renewal by pre-existing cardiomyocytes. Nature. 2013;493:433–6.
Nian M, Lee P, Khaper N, Liu P. Inflammatory cytokines and postmyocardial infarction remodeling. Circ Res. 2004;94:1543–53.
Frangogiannis NG, Dewald O, Xia Y, Ren G, Haudek S, Leucker T, et al. Critical role of monocyte chemoattractant protein-1/CC chemokine ligand 2 in the pathogenesis of ischemic cardiomyopathy. Circulation. 2007;115:584–92.
Georgakis MK, Bernhagen J, Heitman LH, Weber C, Dichgans M. Targeting the CCL2-CCR2 axis for atheroprotection. Eur Heart J. 2022;43:1799–808.
Dewald O, Zymek P, Winkelmann K, Koerting A, Ren G, Abou-Khamis T, et al. CCL2/monocyte chemoattractant protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ Res. 2005;96:881–9.
Zhang W, Zhu T, Chen L, Luo W, Chao J. MCP-1 mediates ischemia-reperfusion-induced cardiomyocyte apoptosis via MCPIP1 and CaSR. Am J Physiol Heart Circ Physiol. 2020;318:H59–H71.
Zhou L, Azfer A, Niu J, Graham S, Choudhury M, Adamski FM, et al. Monocyte chemoattractant protein-1 induces a novel transcription factor that causes cardiac myocyte apoptosis and ventricular dysfunction. Circ Res. 2006;98:1177–85.
Li Y, Feng J, Song S, Li H, Yang H, Zhou B, et al. gp130 controls cardiomyocyte proliferation and heart regeneration. Circulation. 2020;142:967–82.
Miyawaki A, Obana M, Mitsuhara Y, Orimoto A, Nakayasu Y, Yamashita T, et al. Adult murine cardiomyocytes exhibit regenerative activity with cell cycle reentry through STAT3 in the healing process of myocarditis. Sci Rep. 2017;7:1407.
Osugi T, Oshima Y, Fujio Y, Funamoto M, Yamashita A, Negoro S, et al. Cardiac-specific activation of signal transducer and activator of transcription 3 promotes vascular formation in the heart. J Biol Chem. 2002;277:6676–81.
Hilfiker-Kleiner D, Hilfiker A, Drexler H. Many good reasons to have STAT3 in the heart. Pharmacol Ther. 2005;107:131–7.
Hilfiker-Kleiner D, Hilfiker A, Fuchs M, Kaminski K, Schaefer A, Schieffer B, et al. Signal transducer and activator of transcription 3 is required for myocardial capillary growth, control of interstitial matrix deposition, and heart protection from ischemic injury. Circ Res. 2004;95:187–95.
Combadiere C, Potteaux S, Rodero M, Simon T, Pezard A, Esposito B, et al. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6C(hi) and Ly6C(lo) monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation. 2008;117:1649–57.
Owsiany KM, Deaton RA, Soohoo KG, Tram Nguyen A, Owens GK. Dichotomous roles of smooth muscle cell-derived MCP1 (monocyte chemoattractant protein 1) in development of atherosclerosis. Arterioscler Thromb Vasc Biol. 2022;42:942–56.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (81930008, 81900792), the Natural Science Foundation of Chongqing (2023NSCQ-MSX2101), the National Key R&D Program of China (2018YFC1312700), the Program of Innovative Research Team by the National Natural Science Foundation (81721001), and the Program for Changjiang Scholars and Innovative Research Team in University; IRT1216.
Author information
Authors and Affiliations
Contributions
WW, LPL, and CYZ conceived the study, designed the experiments and wrote the manuscript; WW performed immunofluorescence staining, enzyme-linked immunosorbent assays, real-time PCR, and endothelial tube formation assays; XKC generated the animal model and performed Masson staining analysis; LZ and FW performed echocardiography and evaluated heart function; YJH performed western blotting; BJL and ZXL performed data analysis; ZGH performed several experiments related to the animal model; XWX isolated and cultured primary cardiomyocytes; WEW, LPL and CYZ revised the manuscript. All authors approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, W., Chen, Xk., Zhou, L. et al. Chemokine CCL2 promotes cardiac regeneration and repair in myocardial infarction mice via activation of the JNK/STAT3 axis. Acta Pharmacol Sin 45, 728–737 (2024). https://doi.org/10.1038/s41401-023-01198-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-023-01198-0