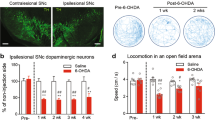
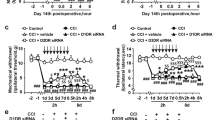
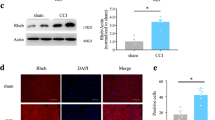
Abstract
Pain is a common annoying non-motor symptom in Parkinson’s disease (PD) that causes distress to patients. Treatment for PD pain remains a big challenge, as its underlying mechanisms are elusive. Pituitary adenylate cyclase-activating polypeptide (PACAP) and its receptor PAC1-R play important roles in regulating a variety of pathophysiological processes. In this study, we investigated whether PACAP/PAC1-R signaling was involved in the mechanisms of PD pain. 6-hydroxydopamine (6-OHDA)-induced PD model was established in rats. Behavioral tests, electrophysiological and Western blotting analysis were conducted 3 weeks later. We found that 6-OHDA rats had significantly lower mechanical paw withdrawal 50% threshold in von Frey filament test and shorter tail flick latency, while mRNA levels of Pacap and Adcyap1r1 (gene encoding PAC1-R) in the spinal dorsal horn were significantly upregulated. Whole-cell recordings from coronal spinal cord slices at L4–L6 revealed that the frequency of spontaneous excitatory postsynaptic currents (sEPSCs) in dorsal horn neurons was significantly increased, which was reversed by application of a PAC1-R antagonist PACAP 6–38 (250 nM). Furthermore, we demonstrated that intrathecal microinjection of PACAP 6–38 (0.125, 0.5, 2 μg) dose-dependently ameliorated the mechanical and thermal hyperalgesia in 6-OHDA rats. Inhibition of PACAP/PAC1-R signaling significantly suppressed the activation of Ca2+/calmodulin-dependent protein kinase II and extracellular signal-regulated kinase (ERK) in spinal dorsal horn of 6-OHDA rats. Microinjection of pAAV-Adcyap1r1 into L4–L6 spinal dorsal horn alleviated hyperalgesia in 6-OHDA rats. Intrathecal microinjection of ERK antagonist PD98059 (10 μg) significantly alleviated hyperalgesia in 6-OHDA rats associated with the inhibition of sEPSCs in dorsal horn neurons. In addition, we found that serum PACAP-38 concentration was significantly increased in PD patients with pain, and positively correlated with numerical rating scale score. In conclusion, activation of PACAP/PAC1-R induces the development of PD pain and targeting PACAP/PAC1-R is an alternative strategy for treating PD pain.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Balestrino R, Schapira AHV. Parkinson disease. Eur J Neurol. 2020;27:27–42.
Bloem BR, Okun MS, Klein C. Parkinson’s disease. Lancet. 2021;397:2284–303.
Silverdale MA, Kobylecki C, Kass-Iliyya L, Martinez-Martin P, Lawton M, Cotterill S, et al. A detailed clinical study of pain in 1957 participants with early/moderate Parkinson’s disease. Parkinsonism Relat Disord. 2018;56:27–32.
Seppi K, Ray Chaudhuri K, Coelho M, Fox SH, Katzenschlager R, Perez Lloret S, et al. Update on treatments for nonmotor symptoms of Parkinson’s disease-an evidence-based medicine review. Mov Disord. 2019;34:180–98.
Campos ACP, Berzuino MB, Hernandes MS, Fonoff ET, Pagano RL. Monoaminergic regulation of nociceptive circuitry in a Parkinson’s disease rat model. Exp Neurol. 2019;318:12–21.
Domenici RA, Campos ACP, Maciel ST, Berzuino MB, Hernandes MS, Fonoff ET, et al. Parkinson’s disease and pain: modulation of nociceptive circuitry in a rat model of nigrostriatal lesion. Exp Neurol. 2019;315:72–81.
Biagioni F, Vivacqua G, Lazzeri G, Ferese R, Iannacone S, Onori P, et al. Chronic MPTP in mice damage-specific neuronal phenotypes within dorsal laminae of the spinal cord. Neurotox Res. 2021;39:156–69.
Denes V, Geck P, Mester A, Gabriel R. Pituitary adenylate cyclase-activating polypeptide: 30 years in research spotlight and 600 million years in service. J Clin Med. 2019;8:1488.
Lam HC, Takahashi K, Ghatei MA, Kanse SM, Polak JM, Bloom SR. Binding sites of a novel neuropeptide pituitary-adenylate-cyclase-activating polypeptide in the rat brain and lung. Eur J Biochem. 1990;193:725–9.
Gottschall PE, Tatsuno I, Miyata A, Arimura A. Characterization and distribution of binding sites for the hypothalamic peptide, pituitary adenylate cyclase-activating polypeptide. Endocrinology. 1990;127:272–7.
Mabuchi T, Shintani N, Matsumura S, Okuda-Ashitaka E, Hashimoto H, Muratani T, et al. Pituitary adenylate cyclase-activating polypeptide is required for the development of spinal sensitization and induction of neuropathic pain. J Neurosci. 2004;24:7283–91.
Sandor K, Kormos V, Botz B, Imreh A, Bolcskei K, Gaszner B, et al. Impaired nocifensive behaviours and mechanical hyperalgesia, but enhanced thermal allodynia in pituitary adenylate cyclase-activating polypeptide deficient mice. Neuropeptides. 2010;44:363–71.
Botz B, Imreh A, Sandor K, Elekes K, Szolcsanyi J, Reglodi D, et al. Role of pituitary adenylate-cyclase activating polypeptide and Tac1 gene derived tachykinins in sensory, motor and vascular functions under normal and neuropathic conditions. Peptides. 2013;43:105–12.
Jongsma H, Pettersson LM, Zhang Y, Reimer MK, Kanje M, Waldenstrom A, et al. Markedly reduced chronic nociceptive response in mice lacking the PAC1 receptor. Neuroreport. 2001;12:2215–9.
Hoffmann J, Miller S, Martins-Oliveira M, Akerman S, Supronsinchai W, Sun H, et al. PAC1 receptor blockade reduces central nociceptive activity: New approach for primary headache? Pain. 2020;161:1670–81.
Ohsawa M, Brailoiu GC, Shiraki M, Dun NJ, Paul K, Tseng LF. Modulation of nociceptive transmission by pituitary adenylate cyclase activating polypeptide in the spinal cord of the mouse. Pain. 2002;100:27–34.
Velasco ER, Florido A, Flores A, Senabre E, Gomez-Gomez A, Torres A, et al. PACAP-PAC1R modulates fear extinction via the ventromedial hypothalamus. Nat Commun. 2022;13:4374.
Baskozos G, Sandy-Hindmarch O, Clark AJ, Windsor K, Karlsson P, Weir GA, et al. Molecular and cellular correlates of human nerve regeneration: ADCYAP1/PACAP enhance nerve outgrowth. Brain. 2020;143:2009–26.
Lee EY, Chan LC, Wang H, Lieng J, Hung M, Srinivasan Y, et al. PACAP is a pathogen-inducible resident antimicrobial neuropeptide affording rapid and contextual molecular host defense of the brain. Proc Natl Acad Sci USA. 2021;118:e1917623117.
Gupte RP, Kadunganattil S, Shepherd AJ, Merrill R, Planer W, Bruchas MR, et al. Convergent phosphomodulation of the major neuronal dendritic potassium channel Kv4.2 by pituitary adenylate cyclase-activating polypeptide. Neuropharmacology. 2016;101:291–308.
Johnson GC, Parsons R, May V, Hammack SE. The role of pituitary adenylate cyclase-activating polypeptide (PACAP) signaling in the hippocampal dentate gyrus. Front Cell Neurosci. 2020;14:111.
Liu NJ, Schnell SA, Schulz S, Wessendorf MW, Gintzler AR. Regulation of spinal dynorphin 1-17 release by endogenous pituitary adenylyl cyclase-activating polypeptide in the male rat: relevance of excitation via disinhibition. J Pharmacol Exp Ther. 2011;336:328–35.
Wang CT, Mao CJ, Zhang XQ, Zhang CY, Lv DJ, Yang YP, et al. Attenuation of hyperalgesia responses via the modulation of 5-hydroxytryptamine signalings in the rostral ventromedial medulla and spinal cord in a 6-hydroxydopamine-induced rat model of Parkinson’s disease. Mol Pain. 2017;13:1–13.
Li CJ, Zhang LG, Liu LB, An MQ, Dong LG, Gu HY, et al. Inhibition of spinal 5-HT3 receptor and spinal dorsal horn neuronal excitability alleviates hyperalgesia in a rat model of Parkinson’s disease. Mol Neurobiol. 2022;59:7253–64.
Buhidma Y, Rukavina K, Chaudhuri KR, Duty S. Potential of animal models for advancing the understanding and treatment of pain in Parkinson’s disease. NPJ Parkinsons Dis. 2020;6:1.
Christensen SL, Hansen RB, Storm MA, Olesen J, Hansen TF, Ossipov M, et al. Von Frey testing revisited: provision of an online algorithm for improved accuracy of 50% thresholds. Eur J Pain. 2020;24:783–90.
Narita M, Dun SL, Dun NJ, Tseng LF. Hyperalgesia induced by pituitary adenylate cyclase-activating polypeptide in the mouse spinal cord. Eur J Pharmacol. 1996;311:121–6.
Cao DL, Zhang ZJ, Xie RG, Jiang BC, Ji RR, Gao YJ. Chemokine CXCL1 enhances inflammatory pain and increases NMDA receptor activity and COX-2 expression in spinal cord neurons via activation of CXCR2. Exp Neurol. 2014;261:328–36.
Li YC, Tian YQ, Wu YY, Xu YC, Zhang PA, Sha J, et al. Upregulation of spinal ASIC1 and NKCC1 expression contributes to chronic visceral pain in rats. Front Mol Neurosci. 2020;13:611179.
Varodayan FP, Minnig MA, Steinman MQ, Oleata CS, Riley MW, Sabino V, et al. PACAP regulation of central amygdala GABAergic synapses is altered by restraint stress. Neuropharmacology. 2020;168:107752.
Yin Y, Huang P, Han Z, Wei G, Zhou C, Wen J, et al. Collagen nanofibers facilitated presynaptic maturation in differentiated neurons from spinal-cord-derived neural stem cells through MAPK/ERK1/2-Synapsin I signaling pathway. Biomacromolecules. 2014;15:2449–60.
Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord. 2015;30:1591–601.
Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, et al. Chronic pain as a symptom or a disease: the IASP classification of chronic pain for the international classification of diseases (ICD-11). Pain. 2019;160:19–27.
Ford B. Pain in Parkinson’s disease. Mov Disord. 2010;25:S98–103.
Chaudhuri KR, Rizos A, Trenkwalder C, Rascol O, Pal S, Martino D, et al. King’s Parkinson’s disease pain scale, the first scale for pain in PD: An international validation. Mov Disord. 2015;30:1623–31.
Johnson GC, May V, Parsons RL, Hammack SE. Parallel signaling pathways of pituitary adenylate cyclase activating polypeptide (PACAP) regulate several intrinsic ion channels. Ann NY Acad Sci. 2019;1455:105–12.
May V, Johnson GC, Hammack SE, Braas KM, Parsons RL. PAC1 receptor internalization and endosomal MEK/ERK activation is essential for PACAP-mediated neuronal excitability. J Mol Neurosci. 2021;71:1536–42.
Yokai M, Kurihara T, Miyata A. Spinal astrocytic activation contributes to both induction and maintenance of pituitary adenylate cyclase-activating polypeptide type 1 receptor-induced long-lasting mechanical allodynia in mice. Mol Pain. 2016;12:1744806916646383.
Faivre F, Sanchez-Catalan MJ, Dovero S, Bido S, Joshi A, Bezard E, et al. Ablation of the tail of the ventral tegmental area compensates symptoms in an experimental model of Parkinson’s disease. Neurobiol Dis. 2020;139:104818.
Qu S, Ondo WG, Zhang X, Xie WJ, Pan TH, Le WD. Projections of diencephalic dopamine neurons into the spinal cord in mice. Exp Brain Res. 2006;168:152–6.
Tang DL, Luan YW, Zhou CY, Xiao C. D2 receptor activation relieves pain hypersensitivity by inhibiting superficial dorsal horn neurons in parkinsonian mice. Acta Pharmacol Sin. 2021;42:189–98.
Taniguchi W, Nakatsuka T, Miyazaki N, Yamada H, Takeda D, Fujita T, et al. In vivo patch-clamp analysis of dopaminergic antinociceptive actions on substantia gelatinosa neurons in the spinal cord. Pain. 2011;152:95–105.
Zheng Y, Zhang L, Xie J, Shi L. The emerging role of neuropeptides in Parkinson’s disease. Front Aging Neurosci. 2021;13:646726.
Zhang Q, Han X, Wu H, Zhang M, Hu G, Dong Z, et al. Dynamic changes in CGRP, PACAP, and PACAP receptors in the trigeminovascular system of a novel repetitive electrical stimulation rat model: relevant to migraine. Mol Pain. 2019;15:1744806918820452.
Davis-Taber R, Baker S, Lehto SG, Zhong C, Surowy CS, Faltynek CR, et al. Central pituitary adenylate cyclase 1 receptors modulate nociceptive behaviors in both inflammatory and neuropathic pain states. J Pain. 2008;9:449–56.
Del Tredici K, Braak H. Spinal cord lesions in sporadic Parkinson’s disease. Acta Neuropathol. 2012;124:643–64.
Perrotta A, Sandrini G, Serrao M, Buscone S, Tassorelli C, Tinazzi M, et al. Facilitated temporal summation of pain at spinal level in Parkinson’s disease. Mov Disord. 2011;26:442–8.
Charles KA, Naudet F, Bouali-Benazzouz R, Landry M, De Deurwaerdere P, Fossat P, et al. Alteration of nociceptive integration in the spinal cord of a rat model of Parkinson’s disease. Mov Disord. 2018;33:1010–5.
Missig G, Mei L, Vizzard MA, Braas KM, Waschek JA, Ressler KJ, et al. Parabrachial pituitary adenylate cyclase-activating polypeptide activation of amygdala endosomal extracellular signal-regulated kinase signaling regulates the emotional component of pain. Biol Psychiatry. 2017;81:671–82.
Akerman S, Goadsby PJ. Neuronal PAC1 receptors mediate delayed activation and sensitization of trigeminocervical neurons: relevance to migraine. Sci Transl Med. 2015;7:308ra157.
Amin FM, Hougaard A, Schytz HW, Asghar MS, Lundholm E, Parvaiz AI, et al. Investigation of the pathophysiological mechanisms of migraine attacks induced by pituitary adenylate cyclase-activating polypeptide-38. Brain. 2014;137:779–94.
Kammermeier PJ. Endogenous homer proteins regulate metabotropic glutamate receptor signaling in neurons. J Neurosci. 2008;28:8560–7.
Michel S, Itri J, Han JH, Gniotczynski K, Colwell CS. Regulation of glutamatergic signalling by PACAP in the mammalian suprachiasmatic nucleus. BMC Neurosci. 2006;7:15.
May V, Clason TA, Buttolph TR, Girard BM, Parsons RL. Calcium influx, but not intracellular calcium release, supports PACAP-mediated ERK activation in HEK PAC1 receptor cells. J Mol Neurosci. 2014;54:342–50.
Langer I, Jeandriens J, Couvineau A, Sanmukh S, Latek D. Signal transduction by VIP and PACAP receptors. Biomedicines. 2022;10:406.
Zhang Q, Cao DL, Zhang ZJ, Jiang BC, Gao YJ. Chemokine CXCL13 mediates orofacial neuropathic pain via CXCR5/ERK pathway in the trigeminal ganglion of mice. J Neuroinflamm. 2016;13:183.
Stamboulian S, Choi JS, Ahn HS, Chang YW, Tyrrell L, Black JA, et al. ERK1/2 mitogen-activated protein kinase phosphorylates sodium channel Na(v)1.7 and alters its gating properties. J Neurosci. 2010;30:1637–47.
Ermilov LG, Schmalz PF, Miller SM, Szurszewski JH. PACAP modulation of the colon-inferior mesenteric ganglion reflex in the guinea pig. J Physiol. 2004;560:231–47.
Otto C, Kovalchuk Y, Wolfer DP, Gass P, Martin M, Zuschratter W, et al. Impairment of mossy fiber long-term potentiation and associative learning in pituitary adenylate cyclase activating polypeptide type I receptor-deficient mice. J Neurosci. 2001;21:5520–7.
Pugh PC, Jayakar SS, Margiotta JF. PACAP/PAC1R signaling modulates acetylcholine release at neuronal nicotinic synapses. Mol Cell Neurosci. 2010;43:244–57.
Komatsu T, Sakurada C, Sasaki M, Sanai K, Tsuzuki M, Bagetta G, et al. Extracellular signal-regulated kinase (ERK) and nitric oxide synthase mediate intrathecal morphine-induced nociceptive behavior. Neuropharmacology. 2007;52:1237–43.
Paredes S, Cantillo S, Candido KD, Knezevic NN. An association of serotonin with pain disorders and its modulation by estrogens. Int J Mol Sci. 2019;20:5729.
Hu S, Huang S, Ma J, Li D, Zhao Z, Zheng J, et al. Correlation of decreased serum pituitary adenylate cyclase-activating polypeptide and vasoactive intestinal peptide levels with non-motor symptoms in patients with Parkinson’s disease. Front Aging Neurosci. 2021;13:689939.
Han P, Liang W, Baxter LC, Yin J, Tang Z, Beach TG, et al. Pituitary adenylate cyclase-activating polypeptide is reduced in Alzheimer disease. Neurology. 2014;82:1724–8.
Pham D, Polgar B, Toth T, Jungling A, Kovacs N, Balas I, et al. Examination of pituitary adenylate cyclase-activating polypeptide in Parkinson’s disease focusing on correlations with motor symptoms. Geroscience. 2022;44:785–803.
Wong CK, Mak RY, Kwok TS, Tsang JS, Leung MY, Funabashi M, et al. Prevalence, incidence, and factors associated with non-specific chronic low back pain in community-dwelling older adults aged 60 years and older: a systematic review and meta-analysis. J Pain. 2022;23:509–34.
Ha AD, Jankovic J. Pain in Parkinson’s disease. Mov Disord. 2012;27:485–91.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (82071420, 82271279), Jiangsu Provincial Key R&D Program (BE2018658), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. We thank Guang-yin Xu for critical discussions of the data in the manuscript.
Author information
Authors and Affiliations
Contributions
CFL designed the study, and revised the manuscript. FW performed experiments, supervised the experiments and edited the manuscript. CJM collected data and edited the manuscript. LGD performed experiments, collected data, analyzed data and prepared figures and the manuscript. MQA performed experiments, collected data, and prepared figures. HYG, LGZ, CJL, and JBZ performed experiments and analyzed data. All the authors have read and approved the paper. The authors have no conflicts of interest to declare.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dong, Lg., An, Mq., Gu, Hy. et al. PACAP/PAC1-R activation contributes to hyperalgesia in 6-OHDA-induced Parkinson’s disease model rats via promoting excitatory synaptic transmission of spinal dorsal horn neurons. Acta Pharmacol Sin 44, 2418–2431 (2023). https://doi.org/10.1038/s41401-023-01141-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-023-01141-3