Abstract
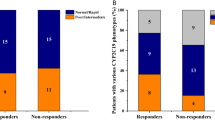
CYP2C19-guided voriconazole dosing reduces pharmacokinetic variability, but many patients remain subtherapeutic. The aim of this study was to evaluate the effect of candidate genes and a novel CYP2C haplotype on voriconazole trough concentrations in patients receiving CYP2C19-guided dosing. This is a retrospective candidate gene study in allogeneic hematopoietic cell transplant (HCT) patients receiving CYP2C19-guided voriconazole dosing. Patients were genotyped for ABCB1, ABCG2, CYP2C9, CYP3A4, CYP3A5, and the CYP2C haplotype. Of 185 patients, 36% were subtherapeutic (of which 79% were normal or intermediate metabolizers). In all patients, CYP2C19 (p < 0.001), age (p = 0.018), and letermovir use (p = 0.001) were associated with voriconazole concentrations. In the subset receiving 200 mg daily (non-RM/UMs), CYP2C19 (p = 0.004) and ABCG2 (p = 0.015) were associated with voriconazole concentrations; CYP2C19 (p = 0.028) and letermovir use (p = 0.001) were associated with subtherapeutic status. CYP2C19 phenotype and letermovir use were significantly associated with subtherapeutic voriconazole concentrations and may be used to improve voriconazole precision dosing, while further research is needed to clarify the role of ABCG2 in voriconazole dosing.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Herbrecht R. Voriconazole: therapeutic review of a new azole antifungal. Expert Rev Anti Infect Ther. 2004;2:485–97.
Smith J, Safdar N, Knasinski V, Simmons W, Bhavnani SM, Ambrose PG, et al. Voriconazole therapeutic drug monitoring. Antimicrob Agents Chemother. 2006;50:1570–2.
Marks DI, Pagliuca A, Kibbler CC, Glasmacher A, Heussel CP, Kantecki M, et al. Voriconazole versus itraconazole for antifungal prophylaxis following allogeneic haematopoietic stem-cell transplantation. Br J Haematol. 2011;155:318–27.
Trifilio S, Ortiz R, Pennick G, Verma A, Pi J, Stosor V, et al. Voriconazole therapeutic drug monitoring in allogeneic hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2005;35:509–13.
Trifilio S, Pennick G, Pi J, Zook J, Golf M, Kaniecki K, et al. Monitoring plasma voriconazole levels may be necessary to avoid subtherapeutic levels in hematopoietic stem cell transplant recipients. Cancer. 2007;109:1532–5.
Theuretzbacher U, Ihle F, Derendorf H. Pharmacokinetic/pharmacodynamic profile of voriconazole. Clin Pharmacokinet. 2006;45:649–63.
Owusu Obeng A, Egelund EF, Alsultan A, Peloquin CA, Johnson JA. CYP2C19 polymorphisms and therapeutic drug monitoring of voriconazole: are we ready for clinical implementation of pharmacogenomics? Pharmacotherapy. 2014;34:703–18.
Moriyama B, Obeng AO, Barbarino J, Penzak SR, Henning SA, Scott SA, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for CYP2C19 and voriconazole therapy. Clin Pharm Ther. 2017;102:45–51.
Hassan A, Burhenne J, Riedel KD, Weiss J, Mikus G, Haefeli WE, et al. Modulators of very low voriconazole concentrations in routine therapeutic drug monitoring. Ther Drug Monit. 2011;33:86–93.
Hamadeh IS, Klinker KP, Borgert SJ, Richards AI, Li W, Mangal N, et al. Impact of the CYP2C19 genotype on voriconazole exposure in adults with invasive fungal infections. Pharmacogenet Genomics. 2017;27:190–6.
Trubiano JA, Crowe A, Worth LJ, Thursky KA, Slavin MA. Putting CYP2C19 genotyping to the test: utility of pharmacogenomic evaluation in a voriconazole-treated haematology cohort. J Antimicrob Chemother. 2015;70:1161–5.
Patel JN, Hamadeh IS, Robinson M, Shahid Z, Symanowski J, Steuerwald N, et al. Evaluation of CYP2C19 genotype-guided voriconazole prophylaxis after allogeneic hematopoietic cell transplant. Clin Pharm Ther. 2020;107:571–9.
Hicks JK, Quilitz RE, Komrokji RS, Kubal TE, Lancet JE, Pasikhova Y, et al. Prospective CYP2C19-guided voriconazole prophylaxis in patients with neutropenic acute myeloid leukemia reduces the incidence of subtherapeutic antifungal plasma concentrations. Clin Pharm Ther. 2020;107:563–70.
Braten LS, Haslemo T, Jukic MM, Ivanov M, Ingelman-Sundberg M, Molden E, et al. A novel CYP2C-haplotype associated with ultrarapid metabolism of escitalopram. Clin Pharm Ther. 2021;110:786–93.
Braten LS, Ingelman-Sundberg M, Jukic MM, Molden E, Kringen MK. Impact of the novel CYP2C:TG haplotype and CYP2B6 variants on sertraline exposure in a large patient population. Clin Transl Sci. 2022;15:2135–45.
Caudle KE, Dunnenberger HM, Freimuth RR, Peterson JF, Burlison JD, Whirl-Carrillo M, et al. Standardizing terms for clinical pharmacogenetic test results: consensus terms from the Clinical Pharmacogenetics Implementation Consortium (CPIC). Genet Med. 2017;19:215–23.
Chuwongwattana S, Jantararoungtong T, Prommas S, Medhasi S, Puangpetch A, Sukasem C. Impact of CYP2C19, CYP3A4, ABCB1, and FMO3 genotypes on plasma voriconazole in Thai patients with invasive fungal infections. Pharm Res Perspect. 2020;8:e00665.
Fan X, Zhang H, Wen Z, Zheng X, Yang Y, Yang J. Effects of CYP2C19, CYP2C9 and CYP3A4 gene polymorphisms on plasma voriconazole levels in Chinese pediatric patients. Pharmacogenet Genomics. 2022;32:152–8.
Allegra S, Fatiguso G, Francia S, Pirro E, Carcieri C, Cusato J, et al. Pharmacogenetic of voriconazole antifungal agent in pediatric patients. Pharmacogenomics. 2018;19:913–25.
Tilen R, Paioni P, Goetschi AN, Goers R, Seibert I, Muller D, et al. Pharmacogenetic analysis of voriconazole treatment in children. Pharmaceutics. 2022;14:1289–302.
Bolcato L, Khouri C, Veringa A, Alffenaar JWC, Yamada T, Naito T, et al. Combined impact of inflammation and pharmacogenomic variants on voriconazole trough concentrations: a meta-analysis of individual data. J Clin Med. 2021;10:2089.
Walsh TJ, Moriyama B, Penzak SR, Klein TE, Caudle KE. Response to “Impact of CYP3A4 genotype on voriconazole exposure: new insights into the contribution of CYP3A4*22 to metabolism of voriconazole”. Clin Pharm Ther. 2018;103:187.
Duflot T, Schrapp A, Bellien J, Lamoureux F. Impact of CYP3A4 genotype on voriconazole exposure. Clin Pharm Ther. 2018;103:185–6.
He HR, Sun JY, Ren XD, Wang TT, Zhai YJ, Chen SY, et al. Effects of CYP3A4 polymorphisms on the plasma concentration of voriconazole. Eur J Clin Microbiol Infect Dis. 2015;34:811–9.
United States Food and Drug Administration. Prevymis prescribing information. 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209939orig1s000,209940orig1s000lbl.pdf.
Nakashima T, Inamoto Y, Fukushi Y, Doke Y, Hashimoto H, Fukuda T, et al. Drug interaction between letermovir and voriconazole after allogeneic hematopoietic cell transplantation. Int J Hematol. 2021;113:872–6.
Hikasa S, Shimabukuro S, Osugi Y, Ikegame K, Kaida K, Fukunaga K, et al. Decrease in voriconazole concentration-to-dose ratio after letermovir initiation: a retrospective, observational study. Bone Marrow Transplant. 2021;56:949–51.
Duong A, Sweet A, Jain R, Hill JA, Pergam SA, Boeckh M, et al. Clinically significant drug interaction: letermovir and voriconazole. J Antimicrob Chemother. 2020;75:775–7.
Author information
Authors and Affiliations
Contributions
JNP, MR, SAM, and EJ wrote the manuscript; JNP, MR, and SAM designed the research; all authors performed the research; JNP, MR, SAM, and EJ analyzed the data; all authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
JNP serves as a paid consultant for VieCure and Clarified Precision Medicine.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Patel, J.N., Robinson, M., Morris, S.A. et al. Pharmacogenetic and clinical predictors of voriconazole concentration in hematopoietic stem cell transplant recipients receiving CYP2C19-guided dosing. Pharmacogenomics J 23, 201–209 (2023). https://doi.org/10.1038/s41397-023-00320-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41397-023-00320-z