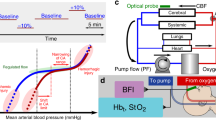
Abstract
Background
Unstable cerebral hemodynamics places preterm infants at high risk of brain injury. We adapted an innovative, fiber-free, wearable diffuse speckle contrast flow-oximetry (DSCFO) device for continuous monitoring of both cerebral blood flow (CBF) and oxygenation in neonatal piglets and preterm infants.
Methods
DSCFO uses two small laser diodes as focused-point and a tiny CMOS camera as a high-density two-dimensional detector to detect spontaneous spatial fluctuation of diffuse laser speckles for CBF measurement, and light intensity attenuations for cerebral oxygenation measurement. The DSCFO was first validated against the established diffuse correlation spectroscopy (DCS) in neonatal piglets and then utilized for continuous CBF and oxygenation monitoring in preterm infants during intermittent hypoxemia (IH) events.
Results
Significant correlations between the DSCFO and DCS measurements of CBF variations in neonatal piglets were observed. IH events induced fluctuations in CBF, cerebral oxygenation, and peripheral cardiorespiratory vitals in preterm infants. However, no consistent correlation patterns were observed among peripheral and cerebral monitoring parameters.
Conclusions
This pilot study demonstrated the feasibility of DSCFO technology to serve as a low-cost wearable sensor for continuous monitoring of multiple cerebral hemodynamic parameters. The results suggested the importance of multi-parameter measurements for understanding deep insights of peripheral and cerebral regulations.
Impact
-
The innovative DSCFO technology may serve as a low-cost wearable sensor for continuous bedside monitoring of multiple cerebral hemodynamic parameters in neonatal intensive care units.
-
Concurrent DSCFO and DCS measurements of CBF variations in neonatal piglet models generated consistent results.
-
No consistent correlation patterns were observed among peripheral and cerebral monitoring parameters in preterm neonates, suggesting the importance of multi-parameter measurements for understanding deep insights of peripheral and cerebral regulations during IH events.
-
Integrating and correlating multiple cerebral functional parameters with clinical outcomes may identify biomarkers for prediction and management of IH associated brain injury.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Perin, J. et al. Global, regional, and national causes of under-5 mortality in 2000–19: an updated systematic analysis with implications for the sustainable development goals. Lancet Child Adolesc. Health 6, 106–115 (2022).
Poets, C. F. Intermittent hypoxia and long-term neurological outcome: how are they related? Semin. Fetal Neonatal Med. 25, 101072 (2020).
Abu Jawdeh, E. G. Intermittent hypoxemia in preterm infants: etiology and clinical relevance. NeoReviews 18, e637–e646 (2017).
Abu Jawdeh, E. G. et al. Intermittent hypoxemia in preterm infants: a potential proinflammatory process. Am. J. Perinatol. 38, 1313–1319 (2020).
Raffay, T. M. et al. Neonatal intermittent hypoxemia events are associated with diagnosis of bronchopulmonary dysplasia at 36 weeks postmenstrual age. Pediatr. Res. 85, 318–323 (2019).
Abu Jawdeh, E. G. Intermittent Hypoxemia in Preterm Infants. Doctoral dissertations, University of Kentucky (2018).
Fiore, J. M. D. et al. Prematurity and postnatal alterations in intermittent hypoxaemia. Arch. Dis. Child. - Fetal Neonatal Ed. 106, 557–559 (2021).
Neubauer, J. A. Invited review: physiological and pathophysiological responses to intermittent hypoxia. J. Appl. Physiol. 90, 1593–1599 (2001).
Chen, L. et al. Oxidative stress and left ventricular function with chronic intermittent hypoxia in rats. Am. J. Respir. Crit. Care Med. 172, 915–920 (2005).
Wong, F. Y. et al. Impaired autoregulation in preterm infants identified by using spatially resolved spectroscopy. Pediatrics 121, e604–e611 (2008).
van Bel, F. & Mintzer, J. P. Monitoring cerebral oxygenation of the immature brain: a neuroprotective strategy? Pediatr. Res. 84, 159–164 (2018).
Verhagen, E. A. et al. Cerebral oxygenation is associated with neurodevelopmental outcome of preterm children at age 2 to 3 years. Dev. Med. Child Neurol. 57, 449–455 (2015).
Di Fiore, J. M., MacFarlane, P. M. & Martin, R. J. Intermittent hypoxemia in preterm infants. Clin. Perinatol. 46, 553–565 (2019).
Gonzalez, C. et al. Arterial Chemoreceptors (Springer, 2009).
Fantini, S., Sassaroli, A., Tgavalekos, K. T. & Kornbluth, J. Cerebral blood flow and autoregulation: current measurement techniques and prospects for noninvasive optical methods. Neurophotonics 3, 031411 (2016).
Kooi, E. M. W. et al. Measuring cerebrovascular autoregulation in preterm infants using near-infrared spectroscopy: an overview of the literature. Expert Rev. Neurother. 17, 801–818 (2017).
Hyttel-Sorensen, S. et al. Cerebral near infrared spectroscopy oximetry in extremely preterm infants: phase II randomised clinical trial. BMJ 350, g7635 (2015).
Diop, M., Kishimoto, J., Toronov, V., Lee, D. S. & St Lawrence, K. Development of a combined broadband near-infrared and diffusion correlation system for monitoring cerebral blood flow and oxidative metabolism in preterm infants. Biomed. Opt. express 6, 3907–3918 (2015).
Andresen, B. et al. Cerebral oxygenation and blood flow in normal term infants at rest measured by a hybrid near-infrared device (BabyLux). Pediatr. Res. 86, 515–521 (2019).
Wyser, D., Lambercy, O., Scholkmann, F., Wolf, M. & Gassert, R. J. N. Wearable and modular functional near-infrared spectroscopy instrument with multidistance measurements at four wavelengths. Neurophotonics 4, 041413–041413 (2017).
Lacerenza, M. et al. Wearable and wireless time-domain near-infrared spectroscopy system for brain and muscle hemodynamic monitoring. Biomed. Opt. express 11, 5934–5949 (2020).
Funane, T. et al. Rearrangeable and exchangeable optical module with system-on-chip for wearable functional near-infrared spectroscopy system. Neurophotonics 5, 011007–011007 (2018).
Roche-Labarbe, N. et al. Noninvasive optical measures of CBV, StO2, CBF index, and rCMRO2 in human premature neonates’ brains in the first six weeks of life. Hum. Brain Mapp. 31, 341–352 (2010).
Buckley, E. M. et al. Cerebral hemodynamics in preterm infants during positional intervention measured with diffuse correlation spectroscopy and transcranial Doppler ultrasound. Opt. Express 17, 12571–12581 (2009).
Shang, Y. et al. Cerebral monitoring during carotid endarterectomy using near-infrared diffuse optical spectroscopies and electroencephalogram. Phys. Med. Biol. 56, 3015 (2011).
Cheng, R., Shang, Y., Hayes, D., Saha, S. P. & Yu, G. Noninvasive optical evaluation of spontaneous low frequency oscillations in cerebral hemodynamics. NeuroImage 62, 1445–1454 (2012).
Sunwoo, J. et al. Diffuse correlation spectroscopy blood flow monitoring for intraventricular hemorrhage vulnerability in extremely low gestational age newborns. Sci. Rep. 12, 12798 (2022).
Farzam, P. et al. Shedding light on the neonatal brain: probing cerebral hemodynamics by diffuse optical spectroscopic methods. Sci. Rep. 7, 15786 (2017).
Shang, Y. et al. Portable optical tissue flow oximeter based on diffuse correlation spectroscopy. Opt. Lett. 34, 3556–3558 (2009).
White, B. R., Liao, S. M., Ferradal, S. L., Inder, T. E. & Culver, J. P. Bedside optical imaging of occipital resting-state functional connectivity in neonates. Neuroimage 59, 2529–2538 (2012).
Frijia, E. M. et al. Functional imaging of the developing brain with wearable high-density diffuse optical tomography: a new benchmark for infant neuroimaging outside the scanner environment. Neuroimage 225, 117490 (2021).
Selb, J. et al. Prolonged monitoring of cerebral blood flow and autoregulation with diffuse correlation spectroscopy in neurocritical care patients. Neurophotonics 5, 045005 (2018).
Liu, X. et al. Simultaneous measurements of tissue blood flow and oxygenation using a wearable fiber-free optical sensor. J. Biomed. Opt. 26, 012705 (2021).
Liu, X. et al. Wearable optical sensor for continuous monitoring of cerebral ischemia in rodents and piglets. In Proc. SPIE PC11956, Biophotonics in Exercise Science, Sports Medicine, Health Monitoring Technologies, and Wearables III. PC1195601 (SPIE, 2022).
Liu, X. et al. A wearable fiber-free optical sensor for continuous monitoring of cerebral blood flow in freely moving mice. In Proc. SPIE 11638, Biophotonics in Exercise Science, Sports Medicine, Health Monitoring Technologies, and Wearables II. 116380A (SPIE, 2021).
Liu, X. et al. A Wearable Fiber-free Optical Sensor for Continuous Monitoring of Cerebral Blood Flow in Freely Behaving Mice. IEEE Transact. Biomed. Eng. 70, 1838–1848 (2023).
Huang, C. et al. A wearable fiberless optical sensor for continuous monitoring of cerebral blood flow in mice. IEEE J. Sel. Top Quantum Electron 25 (2019). https://doi.org/10.1109/JSTQE.2018.2854597
Radlowski, E. C. et al. A neonatal piglet model for investigating brain and cognitive development in small for gestational age human infants. PLoS One 9, e91951 (2014).
Popich, G. A. & Smith, D. W. Fontanels: range of normal size. J. Pediatr. 80, 749–752 (1972).
Vrselja, Z., Brkic, H., Mrdenovic, S., Radic, R. & Curic, G. Function of Circle of Willis. J. Cereb. Blood Flow. Metab. 34, 578–584 (2014).
Kebaya, L. M. N. et al. Three-dimensional cranial ultrasound and functional near-infrared spectroscopy for bedside monitoring of intraventricular hemorrhage in preterm neonates. Sci. Rep. 13, 3730 (2023).
Otic, N. et al. Multi-wavelength multi-distance diffuse correlation spectroscopy system for assessment of premature infants cerebral hemodynamic. In Biophotonics Congress: Biomedical Optics 2022 (Translational, Microscopy, OCT, OTS, BRAIN), Technical Digest Series). Paper JM3A.70 (Optica Publishing Group, 2022).
Huppert, T. J. et al. Sensitivity of neural-hemodynamic coupling to alterations in cerebral blood flow during hypercapnia. J. Biomed. Opt. 14, 044038 (2009).
Rostrup, E., Law, I., Pott, F., Ide, K. & Knudsen, G. M. Cerebral hemodynamics measured with simultaneous PET and near-infrared spectroscopy in humans. Brain Res. 954, 183–193 (2002).
Huang, C. et al. Noninvasive noncontact speckle contrast diffuse correlation tomography of cerebral blood flow in rats. Neuroimage 198, 160–169 (2019).
Acknowledgements
We acknowledge Kimberly Quire and Sara Butler, Department of Pediatrics, University of Kentucky for supporting measurements in NICUs at the Kentucky Children’s Hospital. We also acknowledge Hollie Y. van Rooyen and Jason B. Oakes from the Division of Laboratory Animal Resources at the University of Kentucky for supporting measurements in neonatal piglets.
Funding
Supported partially by the National Institute of Health (NIH, R01-EB028792 to G.Y.; R01-HD101508 to G.Y.; #R56-NS117587 to G.Y.; R21-HD091118 to G.Y.; K23HD109471 to E.G.A.; and UL1-TR001998 to E.G.A.) and University of Kentucky Halcomb Fellowship in Medicine and Engineering to X.L.
Author information
Authors and Affiliations
Contributions
G.Y., Lei.C., E.G.A., H.S.B. and X.L. conceived the study. X.L. designed and built the wearable sensor. X.L., M.M., F.F., S.R.H., P.S. acquired data. Lei.C. conducted and supervised animal experiments. E.G.A. conducted and supervised human experiments. X.L., Li.C. and J.C. analyzed and interpreted data. Li.C. performed statistical analysis. Lei.C. provided resources for animal experiments. E.G.A. and H.S.B. provided resources for human experiments. X.L. drafted the manuscript. M.M., F.F., S.R.H., P.S., G.Y., Lei.C., E.G.A., H.S.B., Li.C., J.C. and X.L. reviewed and edited the manuscript. All authors gave final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
CONSENT STATEMENT
Under a protocol approved by the Institutional Review Board of the University of Kentucky, informed consent was obtained by a physician researcher from each subject’s parents.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Consent Statement Under a protocol approved by the Institutional Review Board of the University of Kentucky, informed consent was obtained by a physician researcher from each subject’s parents.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, X., Mohtasebi, M., Safavi, P. et al. Wearable fiber-free optical sensor for continuous monitoring of neonatal cerebral blood flow and oxygenation. Pediatr Res (2024). https://doi.org/10.1038/s41390-024-03137-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41390-024-03137-z