Abstract
Background
Benign epilepsy with centrotemporal spikes (BECTS) is a common childhood epilepsy syndrome, accompanied by behavioral problems and cognitive impairments. Previous studies of BECTS-related brain structures applied univariate analysis and showed inconsistent results. And neurotransmitter patterns associated with brain structural alterations were still unclear.
Methods
Structural images of twenty-one drug-naïve children with BECTS and thirty-five healthy controls (HCs) were scanned. Segmented gray matter volume (GMV) images were decomposed into independent components (ICs) using the source-based morphometry method. Then spatial correlation analyses were applied to examine possible relationships between GMV changes and neurotransmitter systems.
Results
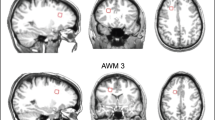
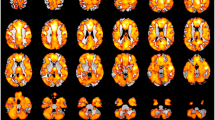
Compared with HCs, drug-naïve children with BECTS showed increased volume in one GMV component (IC7), including bilateral precentral gyrus, bilateral supplementary motor area, left superior frontal cortex, bilateral middle/ inferior frontal cortex and bilateral anterior/ middle cingulate cortex. A positive correlation was observed between one GMV component (IC6) and seizure frequency. There were significantly positive correlations between abnormal GMV in IC7 and serotonergic, GABAergic and glutamatergic systems.
Conclusion
These findings provided further evidence of changed GMV in drug-naïve children with BECTS related to their behavioral problems and cognitive impairments, and associated neurotransmitters which could help to better understand neurobiological mechanisms and underlying molecular mechanisms of BECTS.
Impact
-
The article provides further evidence of changed gray matter volume in drug-naïve children with BECTS related to their behavioral problems and cognitive impairments as well as associated neurotransmitters.
-
Most literature to date has applied univariate analysis and showed inconsistent results, and neurotransmitter patterns associated with brain structural alterations were still unclear. Therefore, this article uses multivariate method and JuSpace toolbox to fill the gap.
-
Significantly increased gray matter volume was found in drug-naïve children with BECTS compared with healthy controls.
-
Abnormal gray matter volume was significantly correlated with clinical data and specific neurotransmitters.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The datasets generated and/or analyzed during this study are available from the corresponding author on reasonable request.
References
Luo, C. et al. Altered structural and functional feature of striato-cortical circuit in Benign Epilepsy with Centrotemporal spikes. Int. J. Neural Syst. 25, 1550027 (2015).
Luo, T. et al. EMD-WOG-2DCNN based EEG signal processing for Rolandic seizure classification. Comput. Methods Biomech. Biomed. Eng. 25, 1565–1575 (2022).
Xiao, F. et al. Altered attention networks in benign childhood epilepsy with centrotemporal spikes (BECTS): A resting-state fMRI study. Epilepsy Behav. 45, 234–241 (2015).
Bourel-Ponchel, E., Mahmoudzadeh, M., Berquin, P. & Wallois, F. Local and distant dysregulation of synchronization around interictal spikes in BECTS. Front. Neurosci. 11, 59 (2017).
Wirrell, E. Benign epilepsy of childhood with centrotemporal spikes. Epilepsia 39, S32–S41 (1998).
Panayiotopoulos, C. P., Michael, M., Sanders, S., Valeta, T. & Koutroumanidis, M. Benign childhood focal epilepsies: assessment of established and newly recognized syndromes. Brain: J. Neurol. 131, 2264–2286 (2008).
Overvliet, G. M. et al. Early onset of cortical thinning in children with rolandic epilepsy. NeuroImage. Clin. 2, 434–439 (2013).
Li, Y. et al. Alterations in the default mode network in rolandic epilepsy with mild spike-wave index in non-rapid eye movement sleep. Front. Neurosci. 16, 944391 (2022).
Fujiwara, H. et al. Cortical and subcortical volume differences between Benign epilepsy with centrotemporal spikes and childhood absence epilepsy. Epilepsy Res. 166, 106407 (2020).
Spencer, E. R. et al. Source EEG reveals that Rolandic epilepsy is a regional epileptic encephalopathy. NeuroImage. Clin. 33, 102956 (2022).
Garcia-Ramos, C. et al. Cognition and brain development in children with benign epilepsy with centrotemporal spikes. Epilepsia 56, 1615–1622 (2015).
Vannest, J., Tenney, J. R., Gelineau-Morel, R., Maloney, T. & Glauser, T. A. Cognitive and behavioral outcomes in benign childhood epilepsy with centrotemporal spikes. Epilepsy Behav. 45, 85–91 (2015).
Kavros, P. M. et al. Attention impairment in rolandic epilepsy: systematic review. Epilepsia 49, 1570–1580 (2008).
Abe, M. Neurophysiological correlates of motor deficits in patients in Benign Epilepsy with Centro-Temporal Spikes. Clin. Neurophysiol. 127, 991–992 (2016).
Salman, R. et al. Brain magnetic resonance imaging findings and brain volumetric differences in a large series of benign rolandic epilepsy. Neuroradiol. J. 35, 692–700 (2022).
Pardoe, H. R., Berg, A. T., Archer, J. S., Fulbright, R. K. & Jackson, G. D. A neurodevelopmental basis for BECTS: evidence from structural MRI. Epilepsy Res. 105, 133–139 (2013).
Kim, E. H. et al. Structural abnormalities in benign childhood epilepsy with centrotemporal spikes (BCECTS). Seizure 27, 40–46 (2015).
Xu, Y. et al. Influence of epileptogenic region on brain structural changes in Rolandic epilepsy. Brain Imaging Behav. 16, 424–434 (2022).
Karalok, Z. S., Ozturk, Z. & Gunes, A. Cortical thinning in benign epilepsy with centrotemporal spikes (BECTS) with or without attention-deficit/hyperactivity (ADHD). J. Clin. Neurosci. 68, 123–127 (2019).
Xu, L., Groth, K. M., Pearlson, G., Schretlen, D. J. & Calhoun, V. D. Source-based morphometry: the use of independent component analysis to identify gray matter differences with application to schizophrenia. Hum. Brain Mapp. 30, 711–724 (2009).
Nguyen, L. et al. Brain structural network alterations related to serum cortisol levels in drug-naive, first-episode major depressive disorder patients: a source-based morphometric study. Sci. Rep. 10, 22096 (2020).
Miola, A. et al. Gray matter volume covariance networks are associated with altered emotional processing in bipolar disorder: a source-based morphometry study. Brain Imaging Behav. 16, 738–747 (2022).
Del Mauro, G. et al. Investigating sexual dimorphism in human brain structure by combining multiple indexes of brain morphology and source-based morphometry. Brain Struct. Funct. 227, 11–21 (2022).
Penzel, N. et al. Association between age of cannabis initiation and gray matter covariance networks in recent onset psychosis. Neuropsychopharmacology 46, 1484–1493 (2021).
Jiang, W. et al. Structural brain alterations and their association with cognitive function and symptoms in Attention-deficit/Hyperactivity Disorder families. NeuroImage. Clin. 27, 102273 (2020).
Quide, Y. et al. Systemic inflammation and grey matter volume in schizophrenia and bipolar disorder: Moderation by childhood trauma severity. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 105, 110013 (2021).
Dukart, J. et al. Cerebral blood flow predicts differential neurotransmitter activity. Sci. Rep. 8, 4074 (2018).
Dukart, J. et al. JuSpace: A tool for spatial correlation analyses of magnetic resonance imaging data with nuclear imaging derived neurotransmitter maps. Hum. Brain Mapp. 42, 555–566 (2021).
Tang, C. et al. The correspondence between morphometric MRI and metabolic profile in Rasmussen’s encephalitis. NeuroImage. Clin. 33, 102918 (2022).
Premi, E. et al. Unravelling neurotransmitters impairment in primary progressive aphasias. Hum. Brain Mapp. 44, 2245–2253 (2023).
Singh, A., Arya, A., Agarwal, V., Shree, R. & Kumar, U. Grey and white matter alteration in euthymic children with bipolar disorder: a combined source-based morphometry (SBM) and voxel-based morphometry (VBM) study. Brain Imaging Behav. 16, 22–30 (2022).
Lapomarda, G. et al. Out of control: An altered parieto-occipital-cerebellar network for impulsivity in bipolar disorder. Behav. Brain Res. 406, 113228 (2021).
Caprihan, A. et al. Source-based morphometry analysis of group differences in fractional anisotropy in schizophrenia. Brain Connect. 1, 133–145 (2011).
Schneider, I. et al. Oxytocin modulates intrinsic neural activity in patients with chronic low back pain. Eur. J. Pain. 24, 945–955 (2020).
Yarkoni, T., Poldrack, R., Nichols, T., Van Essen, D. & Wager, T. Large-scale automated synthesis of human functional neuroimaging data. Nat. Methods 8, 665–670 (2011).
Hansen, J. Y. et al. Mapping neurotransmitter systems to the structural and functional organization of the human neocortex. Nat. Neurosci. 25, 1569–1581 (2022).
Markello, R. et al. neuromaps: structural and functional interpretation of brain maps. Nat. Methods 19, 1472–1479 (2022).
Lenroot, R. K. & Giedd, J. N. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci. Biobehav. Rev. 30, 718–729 (2006).
Laplane, D., Talairach, J., Meininger, V., Bancaud, J. & Orgogozo, J. Clinical consequences of corticectomies involving the supplementary motor area in man. J. Neurol. Sci. 34, 301–314 (1977).
Jin, G. et al. Repetitive transcranial magnetic stimulation to treat benign epilepsy with centrotemporal spikes. Brain Stimul. 15, 601–604 (2022).
Verrotti, A., Filippini, M., Matricardi, S., Agostinelli, M. F. & Gobbi, G. Memory impairment and Benign Epilepsy with centrotemporal spike (BECTS): a growing suspicion. Brain Cogn. 84, 123–131 (2014).
Li, Z. et al. Surface-based morphometry study of the brain in benign childhood epilepsy with centrotemporal spikes. Ann. Transl. Med. 8, 1150 (2020).
Xu, K. et al. Abnormal percent amplitude of fluctuation and functional connectivity within and between networks in benign epilepsy with centrotemporal spikes. Epilepsy Res. 185, 106989 (2022).
Ookawa, S. et al. Frontal fibers connecting the superior frontal Gyrus to Broca Area: A Corticocortical evoked potential study. World Neurosurg. 107, 239–248 (2017).
Yue, Q., Zhang, L., Xu, G., Shu, H. & Li, P. Task-modulated activation and functional connectivity of the temporal and frontal areas during speech comprehension. Neuroscience 237, 87–95 (2013).
Briggs, R. et al. Anatomy and white matter connections of the middle frontal Gyrus. World Neurosurg. 150, e520–e529 (2021).
Liakakis, G., Nickel, J. & Seitz, R. Diversity of the inferior frontal gyrus–a meta-analysis of neuroimaging studies. Behav. Brain Res. 225, 341–347 (2011).
Briggs, R. et al. Anatomy and white matter connections of the inferior frontal gyrus. Clin. Anat. 32, 546–556 (2019).
Vannest, J. et al. Changes in functional organization and functional connectivity during story listening in children with benign childhood epilepsy with centro-temporal spikes. Brain Lang. 193, 10–17 (2019).
Zhu, Y. et al. Intrinsic brain activity as a diagnostic biomarker in children with benign epilepsy with centrotemporal spikes. Hum. Brain Mapp. 36, 3878–3889 (2015).
Tang, Y. et al. Altered regional homogeneity in rolandic epilepsy: a resting-state FMRI study. BioMed. Res. Int. 2014, 960395 (2014).
Bush, G., Luu, P. & Posner, M. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci. 4, 215–222 (2000).
Yin, Y. et al. Structural and functional changes in drug-naïve benign childhood epilepsy with centrotemporal spikes and their associated gene expression profiles. Cereb. Cortex 33, 5774–5782 (2023).
Vannest, J. et al. Impact of frequency and lateralization of interictal discharges on neuropsychological and fine motor status in children with benign epilepsy with centrotemporal spikes. Epilepsia 57, e161–e167 (2016).
Bagdy, G., Kecskemeti, V., Riba, P. & Jakus, R. Serotonin and epilepsy. J. Neurochem. 100, 857–873 (2007).
Arbabi Jahan, A., Rad, A., Ghanbarabadi, M., Amin, B. & Mohammad-Zadeh, M. The role of serotonin and its receptors on the anticonvulsant effect of curcumin in pentylenetetrazol-induced seizures. Life Sci. 211, 252–260 (2018).
Akyuz, E. et al. Revisiting the role of neurotransmitters in epilepsy: An updated review. Life Sci. 265, 118826 (2021).
Bozzi, Y., Provenzano, G. & Casarosa, S. Neurobiological bases of autism-epilepsy comorbidity: a focus on excitation/inhibition imbalance. Eur. J. Neurosci. 47, 534–548 (2018).
Acknowledgements
The authors acknowledge and thank wholeheartedly the participants of this study. We truly appreciate the patience of study participants for their valuable information, cooperation, and participation. This study was financially supported by the National Natural Science Foundation of China under (grant No. 82270696); Shaanxi Key Research and Development Program (grant No.2023-YBSF-673); Shaanxi Provincial Key Laboratory of Integrated Acupuncture and Medication (grant No. KF2219); Young Outstanding Scientific and Technological Talent of Guizhou Province (grant No. Qiankehepingtairencai[2021]5620), Key Basic Research Program of Guizhou Province (grant No. Qiankehejichu-ZK[2022]zhongdian 051), Talent Program for Future Famous Clinical Doctors of Zunyi Medical University (grant No. rc220211205), and Science and Technology Program of Xi’an (grant No.23YXYJ0004). The authors would like to express their gratitude for the support of these projects.
Author information
Authors and Affiliations
Contributions
Heng Liu, Hua Yang and Hong Lu were responsible for conception and design of the study; Duoli Chen, Chengxiang Liu and Peng Liu assisted with data analysis and interpretation of findings; Duoli Chen and Peng Liu drafted the manuscript. All authors critically reviewed the content and approved the final version for publication.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent statement
All participants volunteered to participate in the present study with informed consents.
Consent to publish
All the authors agreed to publish this study in this journal.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, H., Chen, D., Liu, C. et al. Brain structural changes and molecular analyses in children with benign epilepsy with centrotemporal spikes. Pediatr Res (2024). https://doi.org/10.1038/s41390-024-03118-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41390-024-03118-2