Abstract
Background
There are limited data on the impact of perinatal inflammation on child neurodevelopment in low-middle income countries and among growth-restricted infants.
Methods
Population-based, prospective birth cohort study of 288 infants from July 2016–March 2017 in Sylhet, Bangladesh. Umbilical cord blood was analyzed for interleukin(IL)-1α, IL-1β, IL-6, IL-8, and C-reactive protein(CRP). Child neurodevelopment was assessed at 24 months with Bayley-III Scales of Infant Development. We determined associations between cord blood inflammation and neurodevelopmental outcomes, controlling for potential confounders.
Results
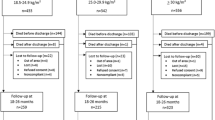
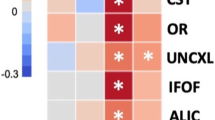
248/288 (86%) live born infants were followed until 24 months, among whom 8.9% were preterm and 45.0% small-for-gestational-age(SGA) at birth. Among all infants, elevated concentrations (>75%) of CRP and IL-6 at birth were associated with increased odds of fine motor delay at 24 months; elevated CRP was also associated with lower receptive communication z-scores. Among SGA infants, elevated IL-1α was associated with cognitive delay, IL-8 with language delay, CRP with lower receptive communication z-scores, and IL-1β with lower expressive communication and motor z-scores.
Conclusions
In rural Bangladesh, perinatal inflammation was associated with impaired neurodevelopment at 24 months. The associations were strongest among SGA infants and noted across several biomarkers and domains, supporting the neurobiological role of inflammation in adverse fetal development, particularly in the setting of fetal growth restriction.
Impact
-
Cord blood inflammation was associated with fine motor and language delays at 24 months of age in a community-based cohort in rural Bangladesh.
-
23.4 million infants are born small-for-gestational-age (SGA) globally each year. Among SGA infants, the associations between cord blood inflammation and adverse outcomes were strong and consistent across several biomarkers and neurodevelopmental domains (cognitive, motor, language), supporting the neurobiological impact of inflammation prominent in growth-restricted infants.
-
Prenatal interventions to prevent intrauterine growth restriction are needed in low- and middle-income countries and may also result in long-term benefits on child development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
United Nations General Assembly. Transforming Our World: The 2030 Agenda for Sustainable Development (A/RES/70/1).; 2015. Accessed August 2, 2023. https://www.refworld.org/docid/57b6e3e44.html.
Blencowe, H. et al. Preterm birth-associated neurodevelopmental impairment estimates at regional and global levels for 2010. Pediatr. Res. 74, 17–34 (2013).
Lee, A. C. C. et al. Intrapartum-related neonatal encephalopathy incidence and impairment at regional and global levels for 2010 with trends from 1990. Pediatr. Res. 74, 50–72 (2013).
Lawn, J. E. et al. Small babies, big risks: global estimates of prevalence and mortality for vulnerable newborns to accelerate change and improve counting. Lancet 401, 1707–1719 (2023).
GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 396, 1204–1222 (2020).
Chico, R. M. et al. Prevalence of malaria and sexually transmitted and reproductive tract infections in pregnancy in sub-Saharan Africa: a systematic review. JAMA 307, 2079–2086 (2012).
Collier, S. A., Rasmussen, S. A., Feldkamp, M. L. & Honein, M. A. National Birth Defects Prevention Study. Prevalence of self-reported infection during pregnancy among control mothers in the National Birth Defects Prevention Study. Birth Defects Res. Part A Clin. Mol. Teratol. 85, 193–201 (2009).
Darmstadt, G. L. et al. 60 Million non-facility births: who can deliver in community settings to reduce intrapartum-related deaths? Int. J. Gynaecol. Obstet. 107, S89–S112 (2009).
Lawn, J. E. et al. Two million intrapartum-related stillbirths and neonatal deaths: where, why, and what can be done? Int. J. Gynaecol. Obstet. 107, S5–S18 (2009). S19.
Black, R. E. et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 382, 427–451 (2013).
Kozuki, N. et al. Short Maternal Stature Increases Risk of Small-for-Gestational-Age and Preterm Births in Low- and Middle-Income Countries: Individual Participant Data Meta-Analysis and Population Attributable Fraction. J. Nutr. 145, 2542–2550 (2015).
Shamim, A. A. et al. Plasma zinc, vitamin B(12) and α-tocopherol are positively and plasma γ-tocopherol is negatively associated with Hb concentration in early pregnancy in north-west Bangladesh. Public Health Nutr. 16, 1354–1361 (2013).
Vohr, B. R., Poggi Davis, E., Wanke, C. A. & Krebs, N. F. Neurodevelopment: The Impact of Nutrition and Inflammation During Preconception and Pregnancy in Low-Resource Settings. Pediatrics 139, S38–S49 (2017).
Méndez Leal, A. S. et al. Maternal early life stress is associated with pro-inflammatory processes during pregnancy. Brain Behav. Immun. 109, 285–291 (2023).
Sävman, K., Blennow, M., Gustafson, K., Tarkowski, E. & Hagberg, H. Cytokine response in cerebrospinal fluid after birth asphyxia. Pediatr. Res. 43, 746–751 (1998).
Foster-Barber, A., Dickens, B. & Ferriero, D. M. Human perinatal asphyxia: correlation of neonatal cytokines with MRI and outcome. Dev. Neurosci. 23, 213–218 (2001).
Leviton, A. et al. The relationship between early concentrations of 25 blood proteins and cerebral white matter injury in preterm newborns: the ELGAN study. J. Pediatr. 158, 897–903.e1 (2011).
Wu, Y. W. et al. Chorioamnionitis and cerebral palsy in term and near-term infants. JAMA 290, 2677–2684 (2003).
Volpe, J. J. Dysmaturation of premature brain: importance, cellular mechanisms, and potential interventions. Pediatr. Neurol. 95, 42–66 (2019).
Liddelow, S. A. et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487 (2017).
Volpe, J. J. Microglia: Newly discovered complexity could lead to targeted therapy for neonatal white matter injury and dysmaturation. J. Neonatal Perinat. Med. 12, 239–242 (2019).
Volpe, J. J., Kinney, H. C., Jensen, F. E. & Rosenberg, P. A. The developing oligodendrocyte: key cellular target in brain injury in the premature infant. Int J. Dev. Neurosci. 29, 423–440 (2011).
Hagberg, H. et al. The role of inflammation in perinatal brain injury. Nat. Rev. Neurol. 11, 192–208 (2015).
Volpe, J. J. Neurobiology of periventricular leukomalacia in the premature infant. Pediatr. Res. 50, 553–562 (2001).
Inder, T. E., Anderson, N. J., Spencer, C., Wells, S. & Volpe, J. J. White matter injury in the premature infant: a comparison between serial cranial sonographic and MR findings at term. AJNR Am. J. Neuroradiol. 24, 805–809 (2003).
Volpe, J. J. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 8, 110–124 (2009).
Dammann, O. & O’Shea, T. M. Cytokines and perinatal brain damage. Clin. Perinatol. 35, 643–663 (2008).
McAdams, R. M. & Juul, S. E. The role of cytokines and inflammatory cells in perinatal brain injury. Neurol. Res. Int. 2012, 561494 (2012).
Back, S. A. White matter injury in the preterm infant: pathology and mechanisms. Acta. Neuropathol. 134, 331–349 (2017).
Kuban, K. C. K. et al. Systemic inflammation and cerebral palsy risk in extremely preterm infants. J. Child Neurol. 29, 1692–1698 (2014).
O’Shea, T. M. et al. Elevated concentrations of inflammation-related proteins in postnatal blood predict severe developmental delay at 2 years of age in extremely preterm infants. J. Pediatr. 160, 395–401.e4 (2012).
O’Shea, T. M. et al. Inflammation-initiating illnesses, inflammation-related proteins, and cognitive impairment in extremely preterm infants. Brain Behav. Immun. 29, 104–112 (2013).
O’Shea, T. M. et al. Elevated blood levels of inflammation-related proteins are associated with an attention problem at age 24 mo in extremely preterm infants. Pediatr. Res. 75, 781–787 (2014).
Leviton, A. et al. Early postnatal blood concentrations of inflammation-related proteins and microcephaly two years later in infants born before the 28th post-menstrual week. Early Hum. Dev. 87, 325–330 (2011).
Bartha, A. I. et al. Neonatal encephalopathy: association of cytokines with MR spectroscopy and outcome. Pediatr. Res. 56, 960–966 (2004).
Lee, A. C. C. et al. National and regional estimates of term and preterm babies born small for gestational age in 138 low-income and middle-income countries in 2010. Lancet Glob. Health 1, e26–e36 (2013).
Arifeen, S. E. et al. The effect of cord cleansing with chlorhexidine on neonatal mortality in rural Bangladesh: a community-based, cluster-randomised trial. Lancet 379, 1022–1028 (2012).
AMANHI (Alliance for Maternal and Newborn Health Improvement). et al. Development and validation of a simplified algorithm for neonatal gestational age assessment - protocol for the Alliance for Maternal Newborn Health Improvement (AMANHI) prospective cohort study. J. Glob. Health 7, 021201 (2017).
Villar, J. et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet 384, 857–868 (2014).
Fichorova, R. N. et al. Maternal microbe-specific modulation of inflammatory response in extremely low-gestational-age newborns. MBio 2, e00280–10 (2011).
Leviton, A. et al. Systemic inflammation on postnatal days 21 and 28 and indicators of brain dysfunction 2years later among children born before the 28th week of gestation. Early Hum. Dev. 93, 25–32 (2016).
Jiang, N. M. et al. Early life inflammation and neurodevelopmental outcome in Bangladeshi infants growing up in adversity. Am. J. Trop. Med. Hyg. 97, 974–979 (2017).
Jiang, N. M. et al. Febrile illness and pro-inflammatory cytokines are associated with lower neurodevelopmental scores in Bangladeshi infants living in poverty. BMC Pediatr. 14, 50 (2014).
Fichorova, R. N. et al. Biological and technical variables affecting immunoassay recovery of cytokines from human serum and simulated vaginal fluid: a multicenter study. Anal. Chem. 80, 4741–4751 (2008).
Hecht, J. L. et al. Relationship between neonatal blood protein concentrations and placenta histologic characteristics in extremely low GA newborns. Pediatr. Res. 69, 68–73 (2011).
Leviton, A. et al. Two-hit model of brain damage in the very preterm newborn: small for gestational age and postnatal systemic inflammation. Pediatr. Res. 73, 362–370 (2013).
Martin, C. R., Bellomy, M., Allred, E. N., Fichorova, R. N. & Leviton, A. Systemic inflammation associated with severe intestinal injury in extremely low gestational age newborns. Fetal. Pediatr. Pathol. 32, 222–234 (2013).
McElrath, T. F., Allred, E. N., Van Marter, L., Fichorova, R. N. & Leviton, A., ELGAN Study Investigators. Perinatal systemic inflammatory responses of growth-restricted preterm newborns. Acta Paediatr. 102, e439–e442 (2013).
Taylor, B. D. et al. Inflammation biomarkers in vaginal fluid and preterm delivery. Hum. Reprod. 28, 942–952 (2013).
Kuban, K. C. K. et al. Association of Circulating Proinflammatory and Anti-inflammatory Protein Biomarkers in Extremely Preterm Born Children with Subsequent Brain Magnetic Resonance Imaging Volumes and Cognitive Function at Age 10 Years. J. Pediatr. 210, 81–90.e3 (2019).
Albers, C. A. & Grieve, A. J. Test Review: Bayley, N. (2006). Bayley Scales of Infant and Toddler Development- Third Edition. San Antonio, TX: Harcourt Assessment. J. Psychoeduc. Assess. 25, 180–190 (2007).
Hamadani, J. D. et al. Randomized controlled trial of the effect of zinc supplementation on the mental development of Bangladeshi infants. Am. J. Clin. Nutr. 74, 381–386 (2001).
Black, M. M. et al. Iron and zinc supplementation promote motor development and exploratory behavior among Bangladeshi infants. Am. J. Clin. Nutr. 80, 903–910 (2004).
Hamadani, J. D., Huda, S. N., Khatun, F. & Grantham-McGregor, S. M. Psychosocial stimulation improves the development of undernourished children in rural Bangladesh. J. Nutr. 136, 2645–2652 (2006).
Tofail, F. et al. The mental development and behavior of low-birth-weight Bangladeshi infants from an urban low-income community. Eur. J. Clin. Nutr. 66, 237–243 (2012).
Hamadani, J. D. et al. Integrating an early childhood development programme into Bangladeshi primary health-care services: an open-label, cluster-randomised controlled trial. Lancet Glob. Health 7, e366–e375 (2019).
Mehrin, S. F. et al. Integrating a Group-Based, Early Childhood Parenting Intervention Into Primary Health Care Services in Rural Bangladesh: A Cluster-Randomized Controlled Trial. Front Pediatr. 10, 886542 (2022).
Tofail, F. et al. An Integrated Mother-Child Intervention on Child Development and Maternal Mental Health. Pediatrics 151, e2023060221G (2023).
Pendergast, L. L. et al. Assessing development across cultures: Invariance of the Bayley-III Scales Across Seven International MAL-ED sites. Sch. Psychol. Q. 33, 604–614 (2018).
Nahar, B. et al. Early childhood development and stunting: Findings from the MAL-ED birth cohort study in Bangladesh. Matern. Child Nutr. 16, e12864 (2020).
Firth, D. Bias reduction of maximum likelihood estimates. Biometrika 80, 27–38 (1993).
Lee, A. C. et al. Maternal diet, infection, and risk of cord blood inflammation in the Bangladesh Projahnmo pregnancy cohort. Nutrients 13, 3792 (2021).
Colonna, M. & Butovsky, O. Microglia function in the central nervous system during health and neurodegeneration. Annu. Rev. Immunol. 35, 441–468 (2017).
Hansen-Pupp, I. et al. Inflammation at birth is associated with subnormal development in very preterm infants. Pediatr. Res. 64, 183–188 (2008).
Liu, C., Chen, Y., Zhao, D., Zhang, J. & Zhang, Y. Association between funisitis and childhood intellectual development: A prospective cohort study. Front Neurol. 10, 612 (2019).
Badawi, N. et al. Antepartum risk factors for newborn encephalopathy: the Western Australian case-control study. BMJ 317, 1549–1553 (1998).
McIntyre, S., Blair, E., Badawi, N., Keogh, J. & Nelson, K. B. Antecedents of cerebral palsy and perinatal death in term and late preterm singletons. Obstet. Gynecol. 122, 869–877 (2013).
Sacchi, C. et al. Association of Intrauterine Growth Restriction and Small for Gestational Age Status With Childhood Cognitive Outcomes: A Systematic Review and Meta-analysis. JAMA Pediatr. 174, 772–781 (2020).
Levine, T. A. et al. Early childhood neurodevelopment after intrauterine growth restriction: a systematic review. Pediatrics 135, 126–141 (2015).
Burton, G. J. & Jauniaux, E. Pathophysiology of placental-derived fetal growth restriction. Am. J. Obstet. Gynecol. 218, S745–S761 (2018).
Wu B. A. et al. Effects of fetal growth restriction on the perinatal neurovascular unit and possible treatment targets. Pediatr Res. Published online September 6. https://doi.org/10.1038/s41390-023-02805-w (2023).
Campbell, L. R. et al. Intracerebral lipopolysaccharide induces neuroinflammatory change and augmented brain injury in growth-restricted neonatal rats. Pediatr. Res. 71, 645–652 (2012).
Dubois, J. et al. Primary cortical folding in the human newborn: an early marker of later functional development. Brain 131, 2028–2041 (2008).
Samuelsen, G. B. et al. Severe cell reduction in the future brain cortex in human growth-restricted fetuses and infants. Am. J. Obstet. Gynecol. 197, 56.e1–7 (2007).
Ross, M. M. et al. A randomized controlled trial investigating the impact of maternal dietary supplementation with pomegranate juice on brain injury in infants with IUGR. Sci. Rep. 11, 3569 (2021).
Han, V. X., Patel, S., Jones, H. F. & Dale, R. C. Maternal immune activation and neuroinflammation in human neurodevelopmental disorders. Nat. Rev. Neurol. 17, 564–579 (2021).
Hsiao, E. Y. & Patterson, P. H. Activation of the maternal immune system induces endocrine changes in the placenta via IL-6. Brain Behav. Immun. 25, 604–615 (2011).
Mandal, M., Marzouk, A. C., Donnelly, R. & Ponzio, N. M. Maternal immune stimulation during pregnancy affects adaptive immunity in offspring to promote development of TH17 cells. Brain Behav. Immun. 25, 863–871 (2011).
Fatemi, S. H. et al. Maternal infection leads to abnormal gene regulation and brain atrophy in mouse offspring: implications for genesis of neurodevelopmental disorders. Schizophr. Res. 99, 56–70 (2008).
Meyer, U. et al. Adult behavioral and pharmacological dysfunctions following disruption of the fetal brain balance between pro-inflammatory and IL-10-mediated anti-inflammatory signaling. Mol. Psychiatry 13, 208–221 (2008).
Meyer, U. et al. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J. Neurosci. 26, 4752–4762 (2006).
Garay, P. A., Hsiao, E. Y., Patterson, P. H. & McAllister, A. K. Maternal immune activation causes age- and region-specific changes in brain cytokines in offspring throughout development. Brain Behav. Immun. 31, 54–68 (2013).
Le Ray, I. et al. Changes in maternal blood inflammatory markers as a predictor of chorioamnionitis: a prospective multicenter study. Am. J. Reprod. Immunol. 73, 79–90 (2015).
Romero, R. et al. Clinical chorioamnionitis at term IV: the maternal plasma cytokine profile. J. Perinat. Med. 44, 77–98 (2016).
Kaukola, T. et al. Population cohort associating chorioamnionitis, cord inflammatory cytokines and neurologic outcome in very preterm, extremely low birth weight infants. Pediatr. Res 59, 478–483 (2006).
Malkova, N. V., Yu, C. Z., Hsiao, E. Y., Moore, M. J. & Patterson, P. H. Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain Behav. Immun. 26, 607–616 (2012).
Patterson, P. H. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav. Brain Res. 204, 313–321 (2009).
Graham, A. M. et al. Maternal Systemic Interleukin-6 During Pregnancy Is Associated With Newborn Amygdala Phenotypes and Subsequent Behavior at 2 Years of Age. Biol. Psychiatry 83, 109–119 (2018).
Rudolph, M. D. et al. Maternal IL-6 during pregnancy can be estimated from newborn brain connectivity and predicts future working memory in offspring. Nat. Neurosci. 21, 765–772 (2018).
Spann, M. N., Monk, C., Scheinost, D. & Peterson, B. S. Maternal Immune Activation During the Third Trimester Is Associated with Neonatal Functional Connectivity of the Salience Network and Fetal to Toddler Behavior. J. Neurosci. 38, 2877–2886 (2018).
Sherwood, E. R. & Prough, D. S. Interleukin-8, neuroinflammation, and secondary brain injury. Crit. Care Med. 28, 1221–1223 (2000).
Najati, N., Rafeey, M. & Melekian, T. Comparison of umbilical cord interlukin-8 in low birth weight infants with premature rupture of membranes and intact membranes. Pak. J. Biol. Sci. 12, 1094–1097 (2009).
Carlo, W. A. et al. Cytokines and neurodevelopmental outcomes in extremely low birth weight infants. J. Pediatr. 159, 919–25.e3 (2011).
Bach, A. M. et al. Systemic inflammation during the first year of life is associated with brain functional connectivity and future cognitive outcomes. Dev. Cogn. Neurosci. 53, 101041 (2022).
Rothman, K. J. No adjustments are needed for multiple comparisons. Epidemiology 1, 43–46 (1990).
Kuban, K. C. K. et al. The breadth and type of systemic inflammation and the risk of adverse neurological outcomes in extremely low gestation newborns. Pediatr. Neurol. 52, 42–48 (2015).
Rogawski McQuade, E. T. et al. Impact of Shigella infections and inflammation early in life on child growth and school-aged cognitive outcomes: Findings from three birth cohorts over eight years. PLoS Negl. Trop. Dis. 16, e0010722 (2022).
Acknowledgements
We would like to thank the Projahnmo research and field staff for implementing the study, and the mothers and children who participated in the study. Dr. Lee would like to thank K23 mentors and advisors (Terrie Inder, Abdullah Baqui, Raina Fichorova, Charles Nelson, Emily Oken and Wafaie Fawzi), the WHO AMANHI Study group, and Tessa Kehoe for assisting with formatting the manuscript for submission. This research was funded by the National Institutes of Child Health and Development (grant number 5K23HD091390). This work co-funded by the Bill & Melinda Gates Foundation through a grant to the World Health Organization/Johns Hopkins University.
Author information
Authors and Affiliations
Contributions
A.C.L., A.H.B., T.I., C.A.N., E.O., and R.F. contributed to the conceptualization and design of the study. F.T., S.R., S.A., R.K., A.H.B., and A.C.L. contributed to acquisition of data. R.F. conducted laboratory analyses. N.H.C. and R.K. contributed to data curation. S.C., A.C.L., L.V.F., and I.O. contributed to the analysis and interpretation of data. A.C.L. drafted the manuscript. A.C.L., S.C., F.T., L.V.F., S.A., S.R., N.H.C., R.K., I.O., E.O., R.F., C.A.N, A.H.B, and T.I. critically revised the manuscript for important intellectual content. All authors have approved of the final version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Informed consent
Informed consent was obtained from all subjects involved in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, A.C., Cherkerzian, S., Tofail, F. et al. Perinatal inflammation, fetal growth restriction, and long-term neurodevelopmental impairment in Bangladesh. Pediatr Res (2024). https://doi.org/10.1038/s41390-024-03101-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41390-024-03101-x