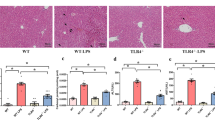
Abstract
Background
The developmental immaturity of the innate immune system helps explains the increased risk of infection in the neonatal period. Importantly, innate immune signaling pathways such as p65/NFκB and c-Jun/AP1 are responsible for the prevention of hepatocyte apoptosis in adult animals, yet whether developmental immaturity of these pathways increases the risk of hepatic injury in the neonatal period is unknown.
Methods
Using a murine model of endotoxemia (LPS 5 mg/kg IP x 1) in neonatal (P3) and adult mice, we evaluated histologic evidence of hepatic injury and apoptosis, presence of p65/NFκB and c-Jun/AP1 activation and associated transcriptional regulation of apoptotic genes.
Results
We demonstrate that in contrast to adults, endotoxemic neonatal (P3) mice exhibit a significant increase in hepatic apoptosis. This is associated with absent hepatic p65/NFκB signaling and impaired expression of anti-apoptotic target genes. Hepatic c-Jun/AP1 activity was attenuated in endotoxemic P3 mice, with resulting upregulation of pro-apoptotic factors.
Conclusions
These results demonstrate that developmental absence of innate immune p65/NFκB and c-Jun/AP1 signaling, and target gene expression is associated with apoptotic injury in neonatal mice. More work is needed to determine if this contributes to long-term hepatic dysfunction, and whether immunomodulatory approaches can prevent this injury.
Impact
-
Various aspects of developmental immaturity of the innate immune system may help explain the increased risk of infection in the neonatal period.
-
In adult models of inflammation and infection, innate immune signaling pathways such as p65/NFκB and c-Jun/AP1 are responsible for a protective, pro-inflammatory transcriptome and regulation of apoptosis.
-
We demonstrate that in contrast to adults, endotoxemic neonatal (P3) mice exhibit a significant increase in hepatic apoptosis associated with absent hepatic p65/NFκB signaling and c-Jun/AP1 activity.
-
We believe that these results may explain in part hepatic dysfunction with neonatal sepsis, and that there may be unrecognized developmental and long-term hepatic implications of early life exposure to systemic inflammatory stress.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data, analytic methods and study materials will be made available to other researchers upon request.
References
Fleischmann-Struzek, C. et al. The global burden of paediatric and neonatal sepsis: a systematic review. Lancet Respir. Med 6, 223–230 (2018).
Schlapbach, L. J. et al. Impact of sepsis on neurodevelopmental outcome in a Swiss national cohort of extremely premature infants. Pediatrics 128, e348–e357 (2011).
Kollmann, T. R., Kampmann, B., Mazmanian, S. K., Marchant, A. & Levy, O. Protecting the newborn and young infant from infectious diseases: lessons from immune ontogeny. Immunity 46, 350–363 (2017).
Sadeghi, K. et al. Immaturity of infection control in preterm and term newborns is associated with impaired toll-like receptor signaling. J. Infect. Dis. 195, 296–302 (2007).
Roger, T. et al. High expression levels of macrophage migration inhibitory factor sustain the innate immune responses of neonates. Proc. Natl Acad. Sci. USA 113, E997–E1005 (2016).
Levy, O. & Wynn, J. L. A prime time for trained immunity: innate immune memory in newborns and infants. Neonatology 105, 136–141 (2014).
Levy, O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat. Rev. Immunol. 7, 379–390 (2007).
Henneke, P., Kierdorf, K., Hall, L. J., Sperandio, M. & Hornef, M. Perinatal development of innate immune topology. Elife 10, e67793 (2021).
Yu, J. C. et al. Innate immunity of neonates and infants. Front Immunol. 9, 1759 (2018).
Strunk, T., Currie, A., Richmond, P., Simmer, K. & Burgner, D. Innate immunity in human newborn infants: prematurity means more than immaturity. J. Matern Fetal Neonatal Med 24, 25–31 (2011).
Zhou, Z., Xu, M. J. & Gao, B. Hepatocytes: a key cell type for innate immunity. Cell Mol. Immunol. 13, 301–315 (2016).
Yan, J., Li, S. & Li, S. The role of the liver in sepsis. Int Rev. Immunol. 33, 498–510 (2014).
Robinson, M. W., Harmon, C. & O’Farrelly, C. Liver immunology and its role in inflammation and homeostasis. Cell Mol. Immunol. 13, 267–276 (2016).
Racanelli, V. & Rehermann, B. The liver as an immunological organ. Hepatology 43, S54–S62 (2006).
Kubes, P. & Jenne, C. Immune responses in the liver. Annu Rev. Immunol. 36, 247–277 (2018).
Knolle, P. A. & Gerken, G. Local control of the immune response in the liver. Immunol. Rev. 174, 21–34 (2000).
Jenne, C. N. & Kubes, P. Immune surveillance by the liver. Nat. Immunol. 14, 996–1006 (2013).
Heymann, F. & Tacke, F. Immunology in the liver–from homeostasis to disease. Nat. Rev. Gastroenterol. Hepatol. 13, 88–110 (2016).
Gao, B., Jeong, W. I. & Tian, Z. Liver: an organ with predominant innate immunity. Hepatology 47, 729–736 (2008).
Strnad, P., Tacke, F., Koch, A. & Trautwein, C. Liver - guardian, modifier and target of sepsis. Nat. Rev. Gastroenterol. Hepatol. 14, 55–66 (2017).
Bode, J. G., Albrecht, U., Häussinger, D., Heinrich, P. C. & Schaper, F. Hepatic acute phase proteins–regulation by Il-6- and Il-1-type cytokines involving stat3 and its crosstalk with NF-κB-dependent signaling. Eur. J. Cell Biol. 91, 496–505 (2012).
Crispe, I. N. Hepatocytes as immunological agents. J. Immunol. 196, 17–21 (2016).
Gabay, C. & Kushner, I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med 340, 448–454 (1999).
Quinton, L. J., Jones, M. R., Robson, B. E. & Mizgerd, J. P. Mechanisms of the hepatic acute-phase response during bacterial pneumonia. Infect. Immun. 77, 2417–2426 (2009).
Quinton, L. J. et al. Hepatocyte-specific mutation of both NF-kappaB rela and stat3 abrogates the acute phase response in mice. J. Clin. Invest 122, 1758–1763 (2012).
Hilliard, K. L. et al. The lung-liver axis: a requirement for maximal innate immunity and hepatoprotection during pneumonia. Am. J. Respir. Cell Mol. Biol. 53, 378–390 (2015).
Lavon, I. et al. High susceptibility to bacterial infection, but no liver dysfunction, in mice compromised for hepatocyte NF-kappaB activation. Nat. Med 6, 573–577 (2000).
Kim, Y. et al. NF-kappaB rela is required for hepatoprotection during pneumonia and sepsis. Infect. Immun. 87, e00132–19 (2019).
Kilpinen, S., Henttinen, T., Lahdenpohja, N., Hulkkonen, J. & Hurme, M. Signals leading to the activation of NF-kappaB transcription factor are stronger in neonatal than adult T lymphocytes. Scand. J. Immunol. 44, 85–88 (1996).
Vancurova, I., Bellani, P. & Davidson, D. Activation of nuclear factor-kappaB and its suppression by dexamethasone in polymorphonuclear leukocytes: newborn versus adult. Pediatr. Res 49, 257–262 (2001).
Wright, C. J., Zhuang, T., La, P., Yang, G. & Dennery, P. A. Hyperoxia-induced NF-kappaB activation occurs via a maturationally sensitive atypical pathway. Am. J. Physiol. Lung Cell Mol. Physiol. 296, L296–L306 (2009).
Yang, G., Abate, A., George, A. G., Weng, Y. H. & Dennery, P. A. Maturational differences in lung NF-kappaB activation and their role in tolerance to hyperoxia. J. Clin. Invest 114, 669–678 (2004).
Claud, E. C. et al. Developmentally regulated IkappaB expression in intestinal epithelium and susceptibility to flagellin-induced inflammation. Proc. Natl Acad. Sci. USA 101, 7404–7408 (2004).
McKenna, S. et al. Endotoxemia induces IkappaBbeta/NF-kappaB-dependent endothelin-1 expression in hepatic macrophages. J. Immunol. 195, 3866–3879 (2015).
McKenna, S. et al. Perinatal endotoxemia induces sustained hepatic Cox-2 expression through an NfkappaB-dependent mechanism. J. Innate Immun. 8, 386–399 (2016).
Zarate, M. A., Nguyen, L. M., De Dios, R. K., Zheng, L. & Wright, C. J. Maturation of the acute hepatic TLR4/NF-kappaB mediated innate immune response Is P65 dependent in mice. Front Immunol. 11, 1892 (2020).
McKenna, S. et al. Immunotolerant P50/NFkappaB signaling and attenuated hepatic IFNbeta expression increases neonatal sensitivity to endotoxemia. Front Immunol. 9, 2210 (2018).
Wullaert, A., van Loo, G., Heyninck, K. & Beyaert, R. Hepatic tumor necrosis factor signaling and nuclear factor-kappaB: effects on liver homeostasis and beyond. Endocr. Rev. 28, 365–386 (2007).
Luedde, T. & Schwabe, R. F. NF-kappaB in the liver–linking injury, fibrosis and hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 8, 108–118 (2011).
Leist, M. et al. Murine hepatocyte apoptosis induced in vitro and in vivo by TNF-Alpha requires transcriptional arrest. J. Immunol. 153, 1778–1788 (1994).
Lehmann, V., Freudenberg, M. A. & Galanos, C. Lethal toxicity of lipopolysaccharide and tumor necrosis factor in normal and D-galactosamine-treated mice. J. Exp. Med 165, 657–663 (1987).
Nagaki, M. et al. Lethal hepatic apoptosis mediated by tumor necrosis factor receptor, unlike fas-mediated apoptosis, requires hepatocyte sensitization in mice. J. Hepatol. 31, 997–1005 (1999).
Geisler, F., Algul, H., Paxian, S. & Schmid, R. M. Genetic inactivation of Rela/P65 sensitizes adult mouse hepatocytes to TNF-induced apoptosis in vivo and in vitro. Gastroenterology 132, 2489–2503 (2007).
Xu, Y. et al. NF-kappaB inactivation converts a hepatocyte cell line TNF-alpha response from proliferation to apoptosis. Am. J. Physiol. 275, C1058–C1066 (1998).
Alcamo, E. et al. Targeted mutation of TNF receptor I rescues the RelA-deficient mouse and reveals a critical role for Nf-kappaB in leukocyte recruitment. J. Immunol. 167, 1592–1600 (2001).
Luedde, T. et al. Deletion of NEMO/Ikkgamma in liver parenchymal cells causes steatohepatitis and hepatocellular carcinoma. Cancer Cell 11, 119–132 (2007).
Bellas, R. E., FitzGerald, M. J., Fausto, N. & Sonenshein, G. E. Inhibition of NF-kappa B activity induces apoptosis in murine hepatocytes. Am. J. Pathol. 151, 891–896 (1997).
Doi, T. S. et al. Absence of tumor necrosis factor rescues rela-deficient mice from embryonic lethality. Proc. Natl Acad. Sci. USA 96, 2994–2999 (1999).
Rosenfeld, M. E., Prichard, L., Shiojiri, N. & Fausto, N. Prevention of hepatic apoptosis and embryonic lethality in RelA/TNFR-1 double knockout mice. Am. J. Pathol. 156, 997–1007 (2000).
Iimuro, Y. et al. NfkappaB prevents apoptosis and liver dysfunction during liver regeneration. J. Clin. Invest 101, 802–811 (1998).
Chaisson, M. L., Brooling, J. T., Ladiges, W., Tsai, S. & Fausto, N. Hepatocyte-specific inhibition of NF-kappaB leads to apoptosis after TNF treatment, but not after partial hepatectomy. J. Clin. Invest 110, 193–202 (2002).
Bettermann, K. et al. Tak1 suppresses a nemo-dependent but NF-kappaB-independent pathway to liver cancer. Cancer Cell 17, 481–496 (2010).
Luedde, T. et al. Deletion of Ikk2 in hepatocytes does not sensitize these cells to Tnf-induced apoptosis but protects from ischemia/reperfusion injury. J. Clin. Invest 115, 849–859 (2005).
Maeda, S. et al. IKKbeta is required for prevention of apoptosis mediated by cell-bound but not by circulating TNFalpha. Immunity 19, 725–737 (2003).
Luedde, T. et al. Ikk1 and Ikk2 cooperate to maintain bile duct integrity in the liver. Proc. Natl Acad. Sci. USA 105, 9733–9738 (2008).
Yang, G., Madan, A. & Dennery, P. A. Maturational differences in hyperoxic Ap-1 activation in rat lung. Am. J. Physiol. Lung Cell Mol. Physiol. 278, L393–L398 (2000).
Choi, A. M., Sylvester, S., Otterbein, L. & Holbrook, N. J. Molecular responses to hyperoxia in vivo: relationship to increased tolerance in aged rats. Am. J. Respir. Cell Mol. Biol. 13, 74–82 (1995).
Tong, L. et al. Attenuated transcriptional responses to oxidative stress in the aged rat brain. J. Neurosci. Res 70, 318–326 (2002).
Eferl, R. et al. Functions of c-Jun in liver and heart development. J. Cell Biol. 145, 1049–1061 (1999).
Hilberg, F., Aguzzi, A., Howells, N. & Wagner, E. F. c-Jun is essential for normal mouse development and hepatogenesis. Nature 365, 179–181 (1993).
Behrens, A. et al. Impaired postnatal hepatocyte proliferation and liver regeneration in mice lacking c-Jun in the liver. EMBO J. 21, 1782–1790 (2002).
Schreiber, M. et al. Control of cell cycle progression by c-Jun Is P53 dependent. Genes Dev. 13, 607–619 (1999).
Eferl, R. et al. Liver tumor development. c-Jun antagonizes the proapoptotic activity of P53. Cell 112, 181–192 (2003).
Butler, B. et al. Developmentally regulated innate immune NF-kappaB signaling mediates Il-1alpha expression in the perinatal murine lung. Front Immunol. 10, 1555 (2019).
Nguyen, L. et al. The hepatic innate immune response is lobe-specific in a murine model endotoxemia. Innate Immun. 25, 144–154 (2019).
Lanaspa, M. A. et al. Ketohexokinase C blockade ameliorates fructose-induced metabolic dysfunction in fructose-sensitive mice. J. Clin. Invest 128, 2226–2238 (2018).
Sherlock, L. G. et al. Apap-induced IkappaBbeta/NfkappaB signaling drives hepatic Il6 expression and associated sinusoidal dilation. Toxicol. Sci. 185, 158–169 (2022).
Griffith, J. W., Sokol, C. L. & Luster, A. D. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev. Immunol. 32, 659–702 (2014).
Hayden, M. S. & Ghosh, S. Signaling to NF-kappaB. Genes Dev. 18, 2195–2224 (2004).
Arvelo, M. B. et al. A20 protects mice from D-galactosamine/lipopolysaccharide acute toxic lethal hepatitis. Hepatology 35, 535–543 (2002).
Beg, A. A., Sha, W. C., Bronson, R. T., Ghosh, S. & Baltimore, D. Embryonic lethality and liver degeneration in mice lacking the rela component of Nf-Kappa B. Nature 376, 167–170 (1995).
Doi, T. S. et al. Rela-deficient lymphocytes: normal development of T cells and B Cells, impaired production of Iga and Igg1 and reduced proliferative responses. J. Exp. Med 185, 953–961 (1997).
Li, Z. W. et al. The IKKbeta subunit of IkappaB kinase (IKK) Is essential for nuclear factor kappaB activation and prevention of apoptosis. J. Exp. Med 189, 1839–1845 (1999).
Li, Q., Van Antwerp, D., Mercurio, F., Lee, K. F. & Verma, I. M. Severe liver degeneration in mice lacking the ikappaB kinase 2 gene. Science 284, 321–325 (1999).
Makris, C. et al. Female mice heterozygous for IKK gamma/NEMO deficiencies develop a dermatopathy similar to the human X-linked disorder incontinentia pigmenti. Mol. Cell 5, 969–979 (2000).
Tanaka, M. et al. Embryonic lethality, liver degeneration, and impaired NF-kappaB activation in ikk-beta-deficient mice. Immunity 10, 421–429 (1999).
Rudolph, D. et al. Severe liver degeneration and lack of NF-kappaB activation in NEMO/IKKgamma-deficient mice. Genes Dev. 14, 854–862 (2000).
Liu, S. et al. Characterization of Rat Cd14 promoter and its regulation by transcription factors Ap1 and Sp family proteins in hepatocytes. Gene 250, 137–147 (2000).
Iida, A. et al. Hepatocyte nuclear factor-kappa beta (NF-kappaB) activation is protective but is decreased in the cholestatic liver with endotoxemia. Surgery 148, 477–489 (2010).
Hochhauser, E. et al. Bone marrow and nonbone marrow toll like receptor 4 regulate acute hepatic injury induced by endotoxemia. PLoS ONE 8, e73041 (2013).
Takamura, M. et al. An inhibitor of c-Jun Nh2-terminal kinase, SP600125, protects mice from D-galactosamine/lipopolysaccharide-induced hepatic failure by modulating Bh3-only proteins. Life Sci. 80, 1335–1344 (2007).
Ben Ari, Z. et al. Reduced hepatic injury in toll-like receptor 4-deficient mice following d-galactosamine/lipopolysaccharide-induced fulminant hepatic failure. Cell Physiol. Biochem 29, 41–50 (2012).
Trusca, V. G., Fuior, E. V., Kardassis, D., Simionescu, M. & Gafencu, A. V. The opposite effect of c-Jun transcription factor on apolipoprotein E gene regulation in hepatocytes and macrophages. Int J. Mol. Sci. 20, 1471 (2019).
Fischer, M. Census and evaluation of P53 target genes. Oncogene 36, 3943–3956 (2017).
Nakano, K. & Vousden, K. H. Puma, a novel proapoptotic gene, is induced by P53. Mol. Cell 7, 683–P694 (2001).
Tan, S. et al. Fas/fasl mediates NF-kappaBp65/puma-modulated hepatocytes apoptosis via autophagy to drive liver fibrosis. Cell Death Dis. 12, 474 (2021).
Cazanave, S. C. et al. Jnk1-dependent puma expression contributes to hepatocyte lipoapoptosis. J. Biol. Chem. 284, 26591–26602 (2009).
Alcorn, J. M., Fierer, J. & Chojkier, M. The acute-phase response protects mice from d-galactosamine sensitization to endotoxin and tumor necrosis factor-alpha. Hepatology 15, 122–129 (1992).
Libert, C., Van Bladel, S., Brouckaert, P., Shaw, A. & Fiers, W. Involvement of the liver, but not of Il-6, in Il-1-induced desensitization to the lethal effects of tumor necrosis factor. J. Immunol. 146, 2625–2632 (1991).
de la Coste, A. et al. Differential protective effects of Bcl-Xl and Bcl-2 on apoptotic liver injury in transgenic mice. Am. J. Physiol. 277, G702–G708 (1999).
Nagaki, M. et al. Tumor necrosis factor alpha prevents tumor necrosis factor receptor-mediated mouse hepatocyte apoptosis, but not fas-mediated apoptosis: role of nuclear factor-kappaB. Hepatology 32, 1272–1279 (2000).
Jia, F. et al. Nod1 agonist protects against lipopolysaccharide and d-galactosamine-induced fatal hepatitis through the upregulation of A20 expression in hepatocytes. Front Immunol. 12, 603192 (2021).
Lee, E. G. et al. Failure to regulate TNF-induced NF-kappaB and cell death responses in a20-deficient mice. Science 289, 2350–2354 (2000).
Catrysse, L. et al. A20 prevents chronic liver inflammation and cancer by protecting hepatocytes from death. Cell Death Dis. 7, e2250 (2016).
Park, J. M. et al. Signaling pathways and genes that inhibit pathogen-induced macrophage apoptosis–CREB and NF-kappaB as key regulators. Immunity 23, 319–329 (2005).
Okada, S., Zhang, H., Hatano, M. & Tokuhisa, T. A physiologic role of Bcl-Xl induced in activated macrophages. J. Immunol. 160, 2590–2596 (1998).
Yabal, M. et al. Xiap restricts tnf- and Rip3-dependent cell death and inflammasome activation. Cell Rep. 7, 1796–1808 (2014).
Conte, D. et al. Inhibitor of apoptosis protein ciap2 is essential for lipopolysaccharide-induced macrophage survival. Mol. Cell Biol. 26, 699–708 (2006).
Funding
This work was supported by NIH grant R01HD107700 to CJW.
Author information
Authors and Affiliations
Contributions
CJW conception and design of research; MRG, WCM, MS, NB, LZ performed experiments; MRG, WCM, NB, DJO analyzed data; MRG, DJO, CJW interpreted results of experiments; CJW drafted the manuscript; MRG, WCM prepared figures; MRG, WCM, MS, NB, LZ, CJW edited and revised manuscript; CJW approved final version of manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All procedures were approved by the University of Colorado Institutional Animal Care and Use Committee (00457) and performed in compliance with the American Association for Accreditation for Laboratory Animal Care at the Perinatal Research Center at the University of Colorado School of Medicine (Aurora, CO).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Grayck, M.R., McCarthy, W.C., Solar, M. et al. Implications of neonatal absence of innate immune mediated NFκB/AP1 signaling in the murine liver. Pediatr Res (2024). https://doi.org/10.1038/s41390-024-03071-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41390-024-03071-0