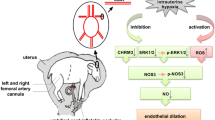
Abstract
Background
Perinatal hypoxia affects a lot of neonates worldwide every year, however its effects on the functioning of systemic circulation are not clear yet. We aimed at investigation the effects of perinatal hypoxia on the second day of life on the functioning of the rat systemic vasculature in early postnatal period.
Methods
2-day-old male rat pups were exposed to normobaric hypoxia (8% O2, 92% N2) for 2 hours. At the 11-14 days cutaneous (saphenous) arteries were isolated and studied by wire myography and Western blotting.
Results
Hypoxia weakened the contribution of anticontractile influence of NO, but did not affect the contribution of Rho-kinase or Kv7 channels to the contraction to α1-adrenergic agonist methoxamine. The content of eNOS and protein kinase G were not altered by hypoxic conditions.
Conclusion
Perinatal hypoxia in rats at the second day of life leads to the decrease of anticontractile effect of NO in the systemic arteries in early postnatal ontogenesis (at the age of 11-14 days). Decreased anticontractile effect of NO can be the reason for insufficient blood supply and represent a risk factor for the development of cardiovascular disorders.
Impact
-
The mechanisms of perinatal hypoxia influences on systemic circulation are almost unknown.
-
We have shown that perinatal hypoxia weakens anticontractile influence of nitric oxide in early postnatal period.
-
The influence of perinatal hypoxia on systemic circulation should be taken into account during treatment of newborns suffered from the lack of oxygen.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Rosa-Mangeret, F. et al. 2.5 million annual deaths—are neonates in low-and middle-income countries too small to be seen? A bottom-up overview on neonatal morbi-mortality. Trop. Med. Infect. Dis. 7, 1–21 (2022).
Barker, D. J. P. Fetal programming of coronary heart disease. Trends Endocrinol. Metab. 13, 364–368 (2002).
Miranda, J. O., Areias, J. C. & Miranda, J. O. Fetal programming as a predictor of adult health or disease: the need to reevaluate fetal heart function. Hear. Fail Rev. 22, 861–877 (2017).
Rood, K. et al. Gestational hypoxia and programing of lung metabolism. Front. Physiol. 10, 1–10 (2019).
Ahearne, C. E., Boylan, G. & Murray, D. Short and long term prognosis in perinatal asphyxia: An update. World J. Clin. Pediatr. 5, 67–74 (2016).
Pospelov, A. S., Puskarjov, M., Kaila, K. & Voipio, J. Endogenous brain-sparing responses in brain pH and PO2 in a rodent model of birth asphyxia. Acta Physiol. 229, e13467 (2020).
Ortiz, M., Loidl, F. & Vázquez-Borsetti, P. Transition to extrauterine life and the modeling of perinatal asphyxia in rats. WIREs Mech. Dis. 14, 1–16 (2022).
Zhao, Y. et al. Hypoxia-induced signaling in the cardiovascular system: pathogenesis and therapeutic targets. Signal Transduct. Target. Ther. 8, 431 (2023).
Gaynullina, D. et al. Functional remodelling of arterial endothelium during early postnatal development in rats. Cardiovasc. Res. 99, 612–621 (2013).
Sofronova, S. I. et al. Endothelial nitric oxide weakens arterial contractile responses and reduces blood pressure during early postnatal development in rats. Nitric Oxide - Biol. Chem. 55–56, 1–9 (2016).
Boegehold, M. A. Endothelium-dependent control of vascular tone during early postnatal and juvenile growth. Microcirculation 17, 394–406 (2010).
Nankervis, C. A. & Nowicki, P. T. Role of nitric oxide in regulation of vascular resistance in postnatal intestine. Am. J. Physiol. 268, 949–958 (1995).
Nankervis, C. A., Dunaway, D. J. & Nowicki, P. T. Determinants of terminal mesenteric artery resistance during the first postnatal month. Am. J. Physiol. - Gastrointest. Liver Physiol. 280, 678–686 (2001).
Shvetsova, A. A., Gaynullina, D. K., Tarasova, O. S. & Schubert, R. Negative feedback regulation of vasocontraction by potassium channels in 10- to 15-day-old rats: Dominating role of Kv7 channels. Acta Physiol. 225, e13176 (2019).
Mochalov, S. V. et al. Higher Ca2+-sensitivity of arterial contraction in 1-week-old rats is due to a greater Rho-kinase activity. Acta Physiol. 223, 1–15 (2018).
Akopov, S. E., Zhang, L. & Pearce, W. J. Regulation of Ca2+ sensitization by PKC and rho proteins in ovine cerebral arteries: Effects of artery size and age. Am. J. Physiol. - Hear. Circ. Physiol. 275, 930–939 (1998).
Braun, D. et al. Hypoxia/reoxygenation of rat renal arteries impairs vasorelaxation via modulation of endothelium-independent sGC/cGMP/PKG signaling. Front. Physiol. 9, 1–11 (2018).
Sedivy, V. et al. Role of Kv7 channels in responses of the pulmonary circulation to hypoxia. Am. J. Physiol. - Lung Cell. Mol. Physiol. 308, L48–L57 (2015).
Lopez, N. C. et al. Role of the RhoA/ROCK pathway in high-altitude associated neonatal pulmonary hypertension in lambs. Am. J. Physiol. - Regul. Integr. Comp. Physiol. 310, R1053–R1063 (2016).
Shvetsova, A. A. et al. Vascular Effects of Perinatal Hypoxia in the Early Postnatal Period in Rats. J. Evol. Biochem. Physiol. 59, 800–808 (2023).
Mulvany, M. J. & Halpern, W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ. Res. 41, 19–26 (1977).
Gaynullina, D. K., Schubert, R. & Tarasova, O. S. Changes in endothelial nitric oxide production in systemic vessels during early ontogenesis—A key mechanism for the perinatal adaptation of the circulatory system. Int. J. Mol. Sci. 20, 1–12 (2019).
Sikarwar, A. S. et al. Hypoxia inhibits adenylyl cyclase catalytic activity in a porcine model of persistent pulmonary hypertension of the newborn. Am. J. Physiol. - Lung Cell. Mol. Physiol. 315, L933–L944 (2018).
de Wijs-Meijler, D. P. M., Duncker, D. J., Danser, A. H. J., Reiss, I. K. M. & Merkus, D. Changes in the nitric oxide pathway of the pulmonary vasculature after exposure to hypoxia in swine model of neonatal pulmonary vascular disease. Physiol. Rep. 6, 1–15 (2018).
Fike, C. D., Slaughter, J. C., Kaplowitz, M. R., Zhang, Y. & Aschner, J. L. Reactive oxygen species from NADPH oxidase contribute to altered pulmonary vascular responses in piglets with chronic hypoxia-induced pulmonary hypertension. Am. J. Physiol. - Lung Cell. Mol. Physiol. 295, 881–888 (2008).
Dennis, K. E. et al. NADPH oxidases and reactive oxygen species at different stages of chronic hypoxia-induced pulmonary hypertension in newborn piglets. Am. J. Physiol. - Lung Cell. Mol. Physiol. 297, L596–L607 (2009).
Fike, C. D. et al. Reactive oxygen species-reducing strategies improve pulmonary arterial responses to nitric oxide in piglets with chronic hypoxia-induced pulmonary hypertension. Antioxid. Redox Signal 18, 1727–1738 (2013).
Wedgwood, S. et al. Increased hydrogen peroxide downregulates soluble guanylate cyclase in the lungs of lambs with persistent pulmonary hypertension of the newborn. Am. J. Physiol. - Lung Cell. Mol. Physiol. 289, 660–666 (2005).
Chen, X. et al. Prenatal hypoxia affected endothelium-dependent vasodilation in mesenteric arteries of aged offspring via increased oxidative stress. Hypertens. Res. 42, 863–875 (2019).
Verschuren, M. T. C., Morton, J. S., Abdalvand, A. & Mansour, Y. The effect of hypoxia-induced intrauterine growth restriction on renal artery function. J. Dev. Orig. Health Dis. 3, 333–341 (2012).
Sugimoto, M. et al. Rho-kinase phosphorylates eNOS at threonine 495 in endothelial cells. Biochem. Biophys. Res. Commun. 361, 462–467 (2007).
Selivanova, E. K., Shvetsova, A. A., Shilova, L. D., Tarasova, O. S. & Gaynullina, D. K. Intrauterine growth restriction weakens anticontractile influence of NO in coronary arteries of adult rats. Sci. Rep. 11, 1–11 (2021).
Lubomirov, L. T. et al. Dual thick and thin filament linked regulation of stretch- and L-NAME-induced tone in young and senescent murine basilar artery. Front. Physiol. 14, 1–18 (2023).
Acknowledgements
The authors thank Dr. E. A. Sebentsova and Dr. N. G. Levitskaya for providing access for facility to model hypoxia. The part of equipment used in the study was provided by MSU within the framework of federal project “The development of infrastructure for science and education” (Agreement \({{{{{\rm{N}}}}}}\underline{{{{{\rm{o}}}}}}\) 161).
Funding
The study was supported by Russian Science Foundation (Project \({{{{{\rm{N}}}}}}\underline{{{{{\rm{o}}}}}}\) 21-75-10036).
Author information
Authors and Affiliations
Contributions
Conceptualization and experimental design (A.A.Sh., D.K.G.), data collection (A.A.Sh., D.D.Kh., S.D.S., M.A.Kh., A.A.B., D.K.G.), data processing (A.A.Sh., D.K.G.), manuscript draft writing (A.A.Sh., D.K.G.).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shvetsova, A.A., Khukhareva, D.D., Simonenko, S.D. et al. Perinatal hypoxia weakens anticontractile influence of NO in rat arteries during early postnatal period. Pediatr Res (2024). https://doi.org/10.1038/s41390-024-03062-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41390-024-03062-1