Abstract
Background
Cardiovascular support (CVS) treatment failure (TF) is associated with a poor prognosis in preterm infants.
Methods
Medical charts of infants with a birth weight <1500 g who received either dopamine (Dp) or dobutamine (Db), were reviewed. Treatment response (TR) occurred if blood pressure increased >3rd centile for gestational age or superior vena cava flow was maintained >55 ml/kg/min, with decreased lactate or less negative base excess, without additional CVS. A predictive model of Dp and Db on TR was designed and the impact of TR on survival was analyzed.
Results
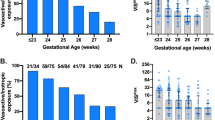
Sixty-six infants (median gestational age 27.3 weeks, median birth weight 864 g) received Dp (n = 44) or Db (n = 22). TR occurred in 59% of the cases treated with Dp and 31% with Db, p = 0.04. Machine learning identified a model that correctly labeled Db response in 90% of the cases and Dp response in 61.4%. Sixteen infants died (9% of the TR group, 39% of the TF group; p = 0.004). Brain or gut morbidity-free survival was observed in 52% vs 30% in the TR and TF groups, respectively (p = 0.08).
Conclusions
New predictive models can anticipate Db but not Dp effectiveness in preterm infants. These algorithms may help the clinicians in the decision-making process.
Impact
-
Failure of cardiovascular support treatment increases the risk of mortality in very low birth weight infants.
-
A predictive model built with machine learning techniques can help anticipate treatment response to dobutamine with high accuracy.
-
Predictive models based on artificial intelligence may guide the clinicians in the decision-making process.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Data for this study are not publicly available as they contain information that could compromise the privacy of the research participants; however, they may be requested upon signing a data access agreement.
References
Aldana-Aguirre, J. C., Deshpande, P., Jain, A. & Weisz, D. E. Physiology of low blood pressure during the first day after birth among extremely preterm neonates. J. Pediatr. 236, 40–46.e3 (2021).
Deshpande, P., Baczynski, M., McNamara, P. J. & Jain, A. Patent ductus arteriosus: the physiology of transition. Semin. Fetal Neonatal Med. 23, 225–231 (2018).
Gephart, S. M. et al. Discrimination of GutCheck NEC: a clinical risk index for necrotizing enterocolitis. J. Perinatol. 34, 468–475 (2014).
Bravo, M. C. et al. Randomized, placebo-controlled trial of dobutamine for low superior vena cava flow in infants. J. Pediatr. 167, 572–8.e1-2 (2015).
Osborn, D. A., Evans, N. & Kluckow, M. Haemodynamic and antecedent risk factors of early and late periventricular/intraventricular hemorrhage in premature infants. Pediatrics 112, 33–39 (2003).
Plomgaard, A. M. et al. The SafeBoosC II randomized trial: treatment guided by near-infrared spectroscopy reduces cerebral hypoxia without changing early biomarkers of brain injury. Pediatr. Res 79, 528–535 (2016).
Baske, K., Saini, S. S., Dutta, S. & Sundaram, V. Epinephrine versus dopamine in neonatal septic shock: a double-blind randomized controlled trial. Eur. J. Pediatr. 177, 1335–1342 (2018).
Dempsey, E. M. et al. Hypotension in Preterm Infants (HIP) randomised trial. Arch. Dis. Child Fetal Neonatal Ed. 106, F398–F403 (2021).
Ismail, R. et al. Methylene blue versus vasopressin analog for refractory septic shock in the preterm neonate: A randomized controlled trial. J. Neonatal Perinat. Med. 15, 265–273 (2022).
Giesinger, R. E. & McNamara, P. J. Haemodynamic instability in the critically ill neonate: an approach to cardiovascular support based on disease pathophysiology. Semin. Perinatol. 40, 174–188 (2016).
Giesinger, R. E., Hobson, A. A., Bischoff, A. R., Klein, J. M. & McNamara, P. J. Impact of early screening echocardiography and targeted PDA treatment on neonatal outcomes in “22-23” week and “24-26” infants. Semin. Perinatol. 47, 151721 (2023).
Miletin, J., Pichova, K. & Dempsey, E. M. Bedside detection of low systemic flow in the very low birth weight infant on day 1 of life. Eur. J. Pediatr. 168, 809–813 (2009).
de Boode, W. P. et al. The role of Neonatologist Performed Echocardiography in the assessment and management of neonatal shock. Pediatr. Res. 84, 78–88 (2018).
Burns, M. L. et al. Inotropic therapy in newborns, A Population-Based National Registry Study. Pediatr. Crit. Care Med. 17, 948–956 (2016).
Pellicer, A. et al. Cardiovascular support for low birth weight infants and cerebral haemodynamics: arandomized, blinded, clinical trial. Pediatrics 115, 1501–1512 (2005)
Pellicer, A. et al. Early systemic hypotension and vasopressor support in low birth weight infants: Impact on neurodevelopment. Pediatrics 123, 1369–1376 (2009).
Ergenekon, E. et al. Cardiovascular drug therapy for human newborn: review of pharmacodynamic data. Curr. Pharm. Des. 23, 5850–5860 (2017).
Osborn, D. A., Evans, N., Kluckow, M., Bowen, J. R. & Rieger, I. Low superior vena cava flow and effect of inotropes on neurodevelopment to 3 years in preterm infants. Pediatrics 120, 372–380 (2007).
Jain, A. et al. Use of targeted neonatal echocardiography to prevent postoperative cardiorespiratory instability after patent ductus arteriosus ligation. J. Pediatr. 160, 584–589 (2012).
Kharrat, A. & Jain, A. Haemodynamic dysfunction in neonatal sepsis. Pediatr. Res. 91, 413–424 (2022).
Kalfa, D., Agrawal, S., Goldshtrom, N., LaPar, D. & Bacha, E. Wireless monitoring and artificial intelligence: A bright future in cardiothoracic surgery. J. Thorac. Cardiovasc Surg. 160, 809–812 (2020).
Krittanawong, C. et al. Future direction for using artificial intelligence to predict and manage hypertension. Curr. Hypertens. Rep. 20, 75 (2018).
Obermeyer, Z. & Emanuel, E. J. Predicting the future — big data, machine learning, and clinical medicine. N. Engl. J. Med. 375, 1216–1219 (2016).
Challen, R. et al. Artificial intelligence, bias and clinical safety. BMJ Qual. Saf. 28, 231–237 (2019).
Yoon Na, J. et al. Artificial intelligence model comparison for risk factor analysis of patent ductus arteriosus in nationwide very low birth weight infants cohort. Sci. Rep. 11, 22353 (2021).
Huang, B. et al. A neonatal dataset and benchmark for non-contact neonatal heart rate monitoring based on spatio-temporal neural networks. Eng. Appl. Artif. Intell. 106, 104447 (2021).
Ghazal S., et al. Using machine learning models to predict oxygen saturation following ventilator support adjustment in critically ill children: a single center pilot study. PLoS ONE 14, e0198921 (2019).
Lin, A. et al. Artificial intelligence: improving the efficiency of cardiovascular imaging. Expert Rev. Med. Devices 17, 565–577 (2020).
Jayakumar, P. et al. Comparison of an artificial intelligence-enabled patient decision aid vs educational material on decision quality, shared decision-making, patient experience, and functional outcomes in adults with knee osteoarthritis: a randomized clinical trial. JAMA Netw. Open 4, e2037107 (2021).
Bravo, M. C. et al. Validity of biomarkers of early circulatory impairment to predict outcome: A retrospective analysis. Front. Pediatr. 7, 212 (2019).
Dempsey, E. M. et al. Hypotension in Preterm Infants (HIP) randomised trial On behalf of the HIP consortium. Arch. Dis. Child Fetal Neonatal Ed. 106, 398–403 (2021).
Agut, T. et al. Preterm white matter injury: ultrasound diagnosis and classification. Pediatr. Res. 87, 37–49 (2020).
Parodi, A. et al. Cranial ultrasound findings in preterm germinal matrix haemorrhage, sequelae and outcome. Pediatr. Res. 87, 13–24 (2020).
McAdams, R. M. et al. Predicting clinical outcomes using artificial intelligence and machine learning in neonatal intensive care units: a systematic review. J. Perinatol. 42, 1561–1575 (2022).
Troyanskaya, O. et al. Missing value estimation methods for DNA microarrays. Bioinformatics 17, 520–525 (2001).
de Boode, W. P. Advanced haemodynamic monitoring in the neonatal intensive care unit. Clin. Perinatol. 47, 423–434 (2020).
Pellicer, A. et al. The SafeBoosC phase II randomised clinical trial: a treatment guideline for targeted near-infrared-derived cerebral tissue oxygenation versus standard treatment in extremely preterm infants. Neonatology 104, 171–178 (2013).
Osborn, D., Evans, N. & Kluckow, M. Randomized trial of dobutamine versus dopamine in preterm infants with low systemic blood flow. J. Pediatr. 140, 183–191 (2002).
Repici, A. et al. Artificial intelligence and colonoscopy experience: Lessons from two randomised trials. Gut 71, 757–765 (2022).
Batton, B. et al. Use of antihypotensive therapies in extremely preterm infants. Pediatrics 131, e1865–e1873 (2013).
Dempsey, E. & Rabe, H. The use of cardiotonic drugs in neonates. Clin. Perinatol. 46, 273–290 (2019).
Paradisis, M., Evans, N., Kluckow, M., Osborn, D. & McLachlan, A. J. Pilot study of milrinone for low systemic blood flow in very preterm infants. J. Pediatr. 148, 306–313 (2006).
Funding
The authors have no potential conflict of interest. The first author, who wrote the first draft, did not receive a fee for producing the manuscript. The corresponding author acknowledges the financial support of the Spanish Health Research Fund (Fondo de Investigación Sanitaria), grant PI22/00567. EP-H acknowledges the support from the Spanish State Research Agency (AEI), project PID2020-115363RB-I00.
Author information
Authors and Affiliations
Contributions
MCB conceptualized and designed the study, drafted the initial manuscript, performed the initial analyses and approved the final manuscript as submitted. RJ collected the patient data, completed the electronic dataset and approved the final manuscript as submitted. EPH designed the data collection instruments, analyzed the data, reviewed the manuscript and approved the final manuscript as submitted. JJF designed the data collection instruments, analyzed the data, reviewed the manuscript and approved the final manuscript as submitted. AP conceptualized and designed the study, drafted the initial manuscript, performed the initial analyses and approved the final manuscript as submitted.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This project received ethical approval from the La Paz University Hospital Ethics Committee. Because it was a retrospective study, patient consent to participate was not necessary.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bravo, M.C., Jiménez, R., Parrado-Hernández, E. et al. Predicting the effectiveness of drugs used for treating cardiovascular conditions in newborn infants. Pediatr Res 95, 1124–1131 (2024). https://doi.org/10.1038/s41390-023-02964-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-023-02964-w